Hanna Instruments HI 83206-2008 User Manual
Page 27
52
53
Dissolved Oxygen
SPECIFICATIONS
Range
0.0 to 10.0 mg/L
Resolution
0.1 mg/L
Accuracy
±0.4 mg/L ±3% of reading
Typical EMC
± 0.1 mg/L
Deviation
Light Source
Tungsten lamp with narrow band interference filter @ 420 nm
Method
Adaptation of the
Standard Methods for the Examination of Water and Wastewater,
18
th
edition, Azide modified Winkler method. The reaction between dissolved oxygen
and the reagents causes a yellow tint in the sample.
REQUIRED REAGENTS
Code
Description
Quantity
HI 93732A-0
Reagent A
5 drops
HI 93732B-0
Reagent B
5 drops
HI 93732C-0
Reagent C
10 drops
REAGENT SET
HI 93732-01 Reagents for 100 tests
HI 93732-03 Reagents for 300 tests
For other accessories see page 73.
MEASUREMENT PROCEDURE
• Select the
Dissolved Oxygen method using the procedure described in
the
Method Selection section (see page 12).
• Fill one 60 mL glass bottle completely with the unreacted sample.
• Replace the cap and ensure that a small part of the sample spills over.
• Remove the cap and add 5 drops of HI 93732A-0 and 5 drops of
HI 93732B-0.
• Add more sample, to fill the bottle completely. Replace the cap again
and ensure that a part of the sample spills over. This is to make sure
that no air bubbles have been trapped inside, which could alter the
reading.
• Invert several times the bottle. The sample becomes orange-yellow and
a flocculent agent will appear.
x 5
x 5
DISSOLVED OXYGEN
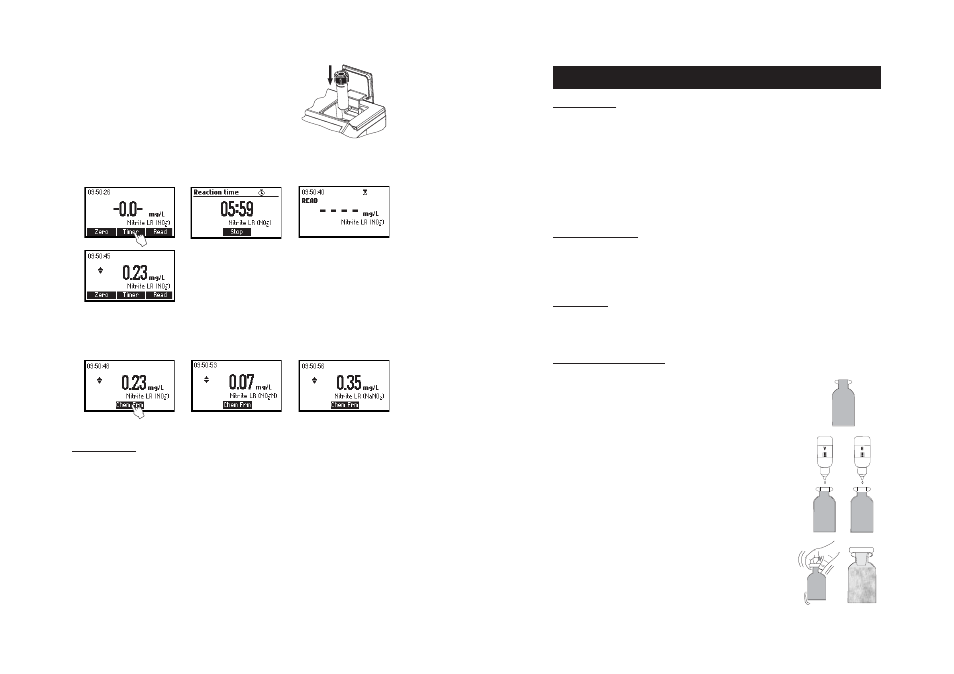
• Reinsert the cuvette into the instrument.
• Press TIMER and the display will show the countdown prior
to the measurement or, alternatively, wait for 6 minutes
and press READ. When the timer ends the meter will perform
the reading. The instrument displays concentration in mg/L
of nitrite.
• Press the or to access the second level of functions.
• Press the Chem Frm functional key to convert the result in mg/L of nitrogen-nitrite (NO
2
–
-N) and sodium
nitrite (NaNO
2
).
• Press the or to go back to the measurement screen.
INTERFERENCES
Interference may be caused by the following ions:
ferrous, ferric, cupric, mercurous, silver, antimonious, bismuth, auric, lead, metavanadate and chloroplatinate.
Strongly reducing and oxidizing reagents.
High levels of nitrate (above 100 mg/L) could yield falsely high readings due to a minute amount of
reduction to nitrite that could occur at these levels.
Nitrite LR
