Mix well then take mv value. 2. 5 ml (vstd) of 10 – Hanna Instruments HI 4010 User Manual
Page 14
14
XII.
XII.
XII.
XII.
XII. Other Measurement T
Other Measurement T
Other Measurement T
Other Measurement T
Other Measurement Techniques
echniques
echniques
echniques
echniques
Known addition (for F
-
)
An unknown concentration can be determined by adding a
known amount (volume and concentration) of measured
ion to a known volume of the sample. This technique is
useful for very low F
-
concentration samples. It can use an
ideal sensor slope, but actual determined slopes at the
temperature of measurement should be used if known.
This method is preprogrammed in the Hanna HI 4222 pH/
ISE/mV meter, which simplifies the method greatly.
Example: Fluoride ion determination with known addition.
1. A 50 mL sample of unknown (Vsample) is placed in
a clean plastic beaker with cleaned electrodes. The
mV 1 is recorded. If fluoride metal complexes are
present add 50 mL TISAB II (V
TISAB
). Mix well then
take mV value.
2. 5 mL (Vstd) of 10
-3
M (Cstd) standard is added to the
beaker and the mV value decreases. The unknown
fluoride concentration in the original sample
(Csample) can then be determined by the following
equation.
3. The procedure can be repeated with a second stan-
dard addition to verify slope and operation of the
method.
(V
sample
+V
standard
+V
ISA
)= V
T
(V
sample
+V
ISA
)= V
S’
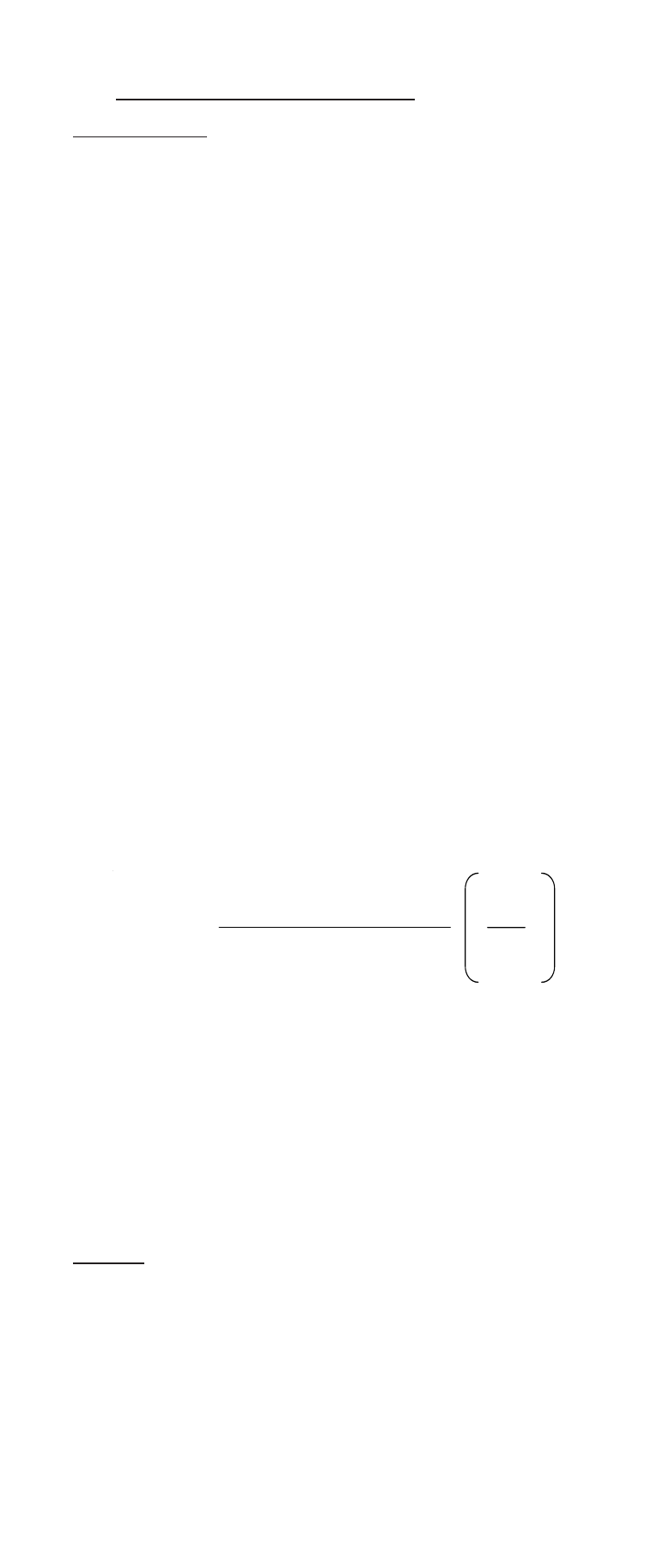
C
sample
=
(V
T
)10
∆E/S
- (V
S’
)
C
standard
V
standard
V
sample
V
S’
Titration
Titration can be used to measure an ion that doesn’t
have an ion selective sensor. An example of this is the use
of the Hanna HI 4110 or HI 4010 fluoride electrode for
aluminum (Al
3+
)
determination. Because the
stoichiometry between the two species is variable fixing
the pH and titrating to a fixed endpoint is advised.
