Bio-Rad Rotofor® and Mini Rotofor Cells User Manual
Page 46
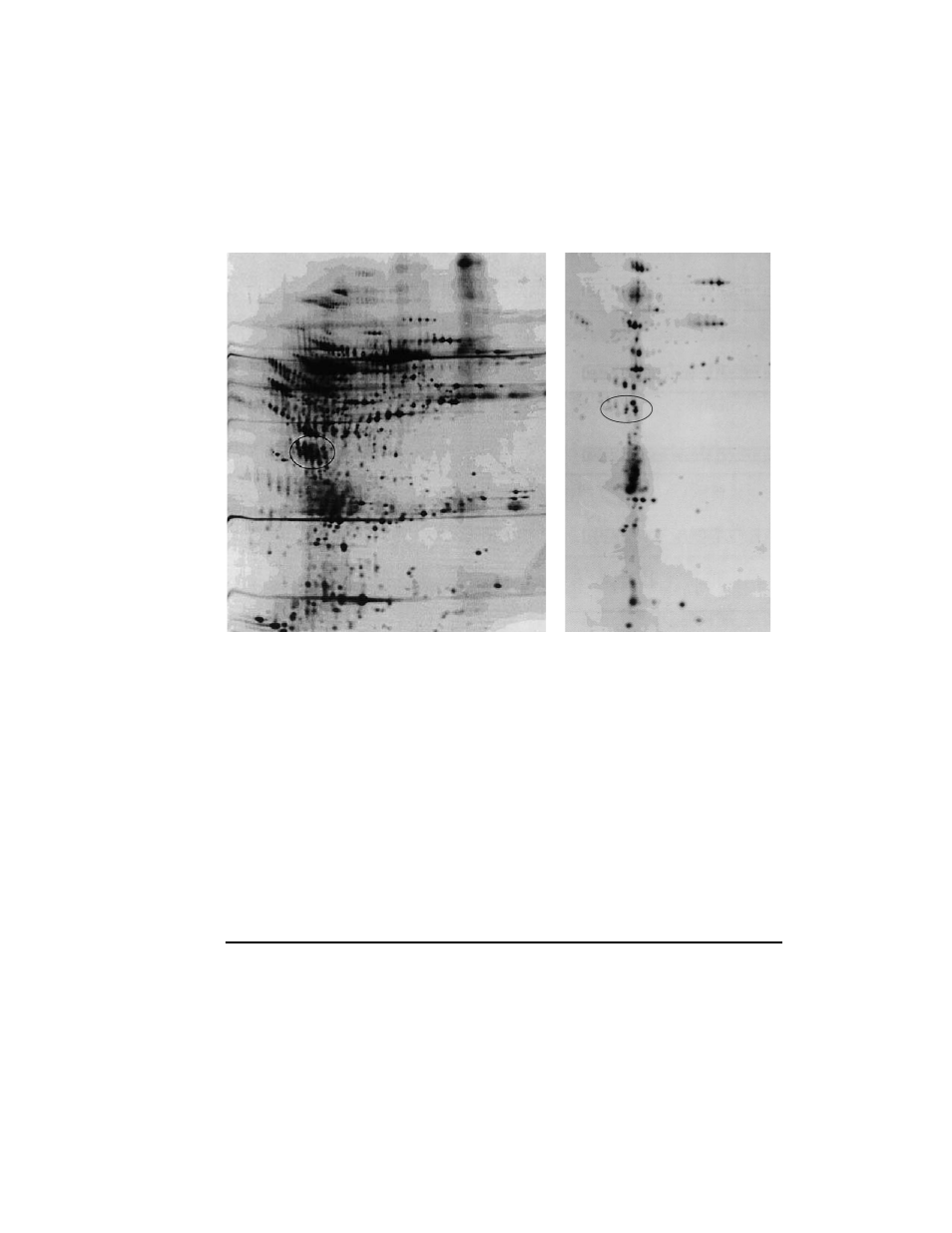
Refractionation
Rotofor fractions 4,5 and 6 were collected, pooled, and refractionated in the
Rotofor cell without additional ampholytes. The total protein load was 50 mil-
ligrams. Upon refractionation, an overall 1000-fold purification of Apo J and the
unknown protein was obtained in Rotofor fraction 11. See Figure 2b.
Fig. 2. Analysis of Rotofor fractions by 2-D gel electrophoresis. 2a) Initial Rotofor fractionation
provided enrichment for the proteins of interest in Rotofor fraction 5, shown here. 2b) Refractionation of
Rotofor fraction 5 shown here resulted in 1000-fold purification of Apo J and the unknown 49 kd protein
by comparison to the starting sample in Figure 1. Analytical 2-D gels were silver stained.
Preparative SDS-PAGE
For preparative gel electrophoresis, the discontinuous buffer system of
Laemmli was used
5
. The total acrylamide concentration (%T) of the separating gel
was optimized at 12%.
The sample (Rotofor fraction 11) contained approximately 2.5 mg of total protein
dissolved in 2.0 ml of sample buffer (see Table 1). After a 5 minute incubation at
95°C, the sample was loaded onto the Prep cell and the gel run for 16 hours.
Running buffer was pumped through the elution chamber at a rate of 0.5 ml per
minute.
Table 1. Model 491 Prep cell running conditions
Resolving gel:
12% acrylamide/ 2.6% C (PDA crosslinker)
Resolving gel length: 8 cm in 37 mm gel tube
Resolving gel buffer: Tris-HCl (0.375 M) pH 8.8
Stacking gel:
4% T/2.6% C (PDA crosslinker)
Stacking gel buffer:
Tris-HCl (125 mM) pH 6.5
Running buffer:
Tris-Glycine-SDS (25 mM-192mM-0.1%)
42
