LaMotte Water & Soil pH User Manual
Page 6
pH & SOIL:
The pH of soil is critical to the survival of plants living in the soil. Not
only is the pH important in itself, it determines the availability of mineral
nutrients for the plants.
Soil can be divided into three pH categories: acid or “sour” soils, alkaline
or “sweet” soil, and neutral soil. Most plants prefer soil with a pH between
6.0 and 8.0, but some plants thrive in acid soils. Azaleas and
rhododendrons are examples of plants which prefer acid soils.
The pH of soil changes from area to area, but a general rule is that soils in
humid regions are acidic, because of rainfall and the “leaching effect,”
where minerals are removed from soil as water passes through. In dry areas
the soil is normally neutral or slightly alkaline. While acid or alkaline soils
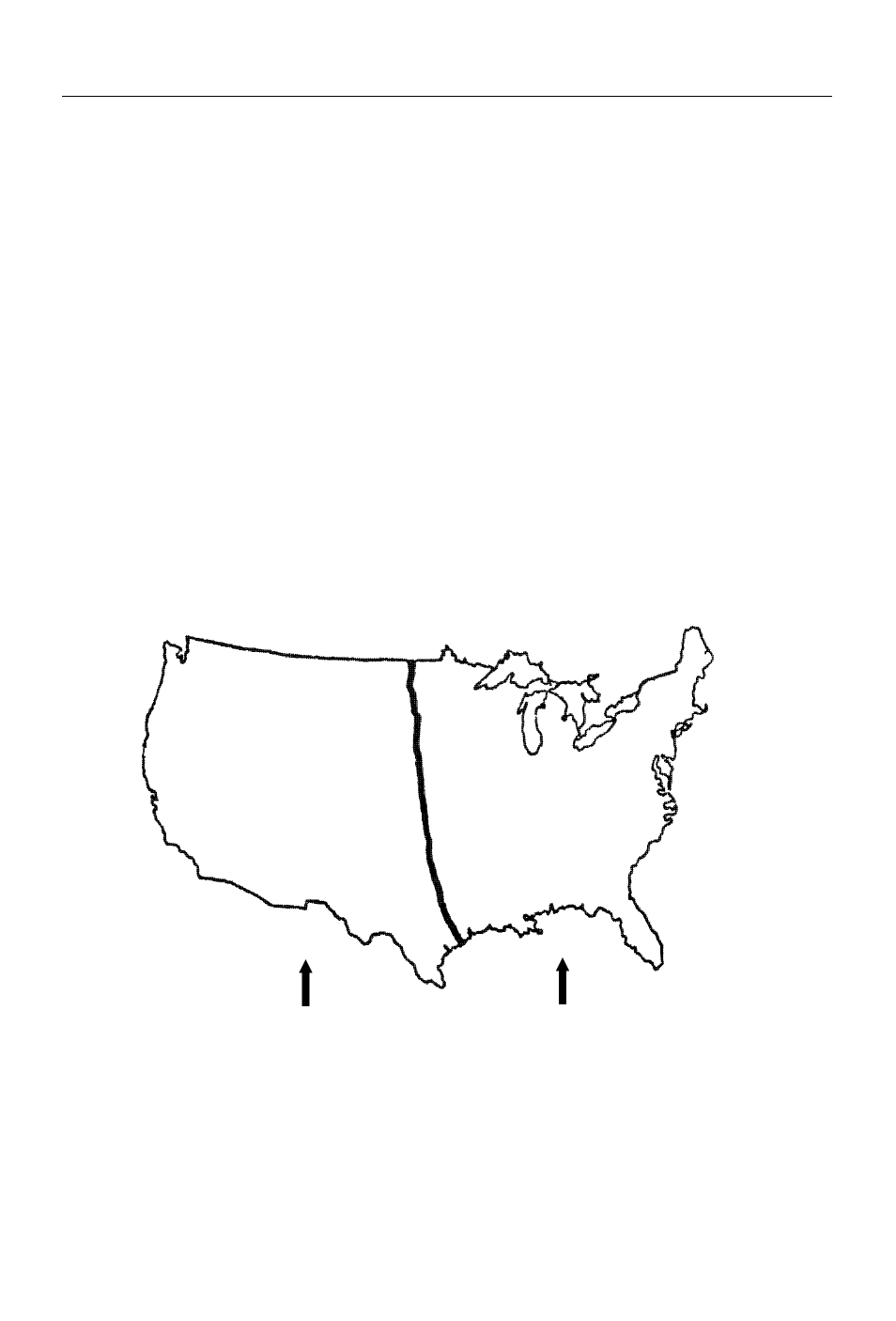
can be found anywhere in the United States, areas east of the Great Plains
generally have acidic soil, while soils west of the Great Plains are usually
neutral or alkaline.
If the pH is outside the desired range, chemicals can be added to adjust the
pH. Lime is often used to raise the pH, and sulfur and alum are commonly
used to lower the pH.
6
Fig. 2 General distribution of soils in the continental
United States in relation to soil reaction.
Neutral and alkaline soils
are common.
Acid soils are common.
