LaMotte Water & Soil pH User Manual
Page 4
What is pH?
One of the simplest, yet most important, analyses of water and soil is the
pH test. pH is a measurement of the concentration of hydrogen ions in a
substance, or how acidic or basic the substance is. The concentration of
hydrogen ions is inversely proportional to the pH; the higher the
concentration of hydrogen ions the lower the pH.
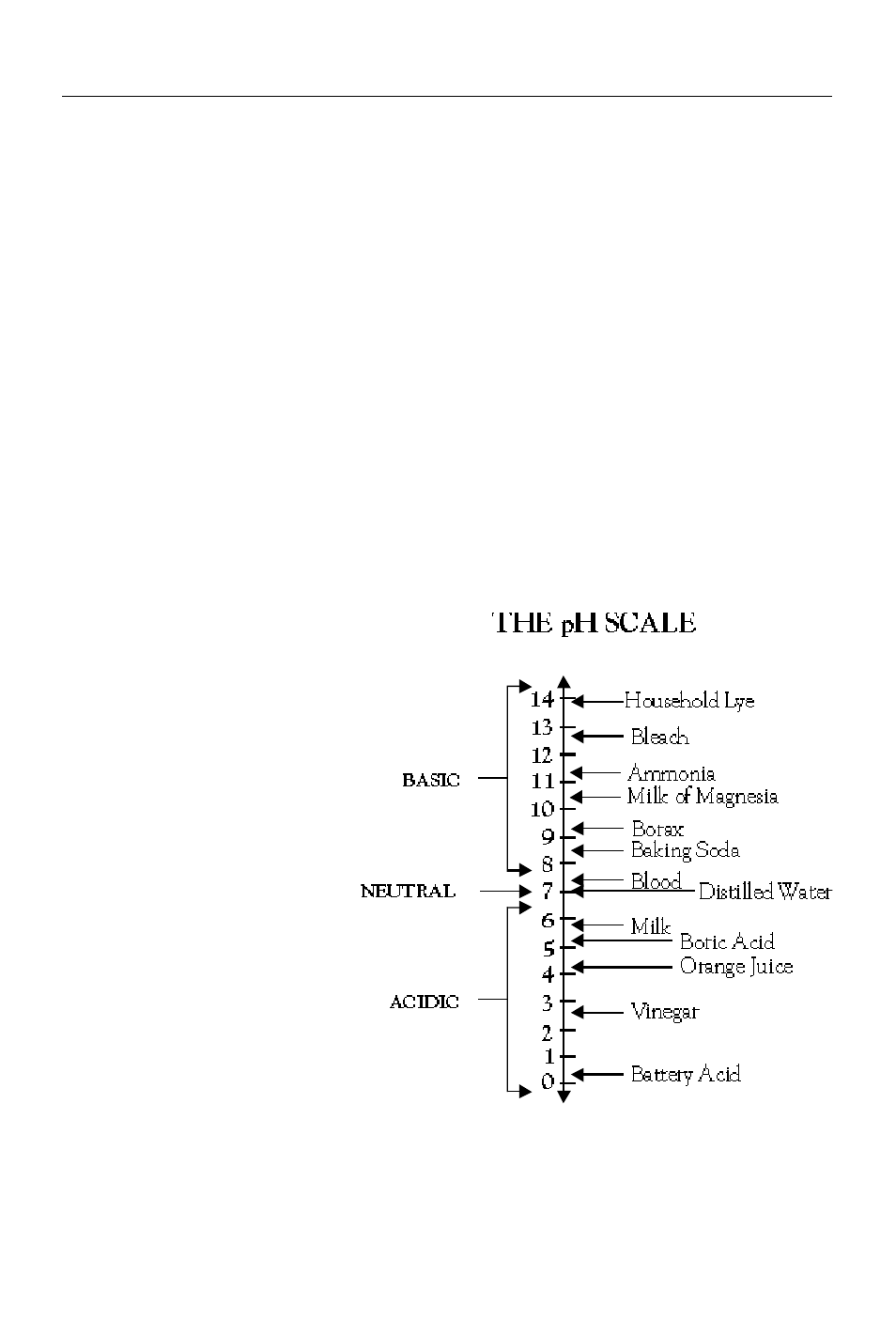
pH is measured on a logarithmic scale which ranges from 0 to 14. A pH of
7.0 is considered neutral; substances with a pH below 7.0 are acidic and
those with a pH above 7.0 are basic, or alkaline. Since the pH scale is
logarithmic, a change of one pH unit reflects a ten fold change in the
acidity. Orange juice, pH 4.0, is ten times more acidic than boric acid, pH
5.0, and 100 times more acidic than milk, pH 6.0. The pH of several
household substances are shown in Fig. 1.
There are many methods which can be used to measure pH. The simplest,
most inexpensive method, is using litmus paper, which, when dipped into
the solution, changes color to indicate whether the solution is acidic,
alkaline or neutral. Litmus paper will only indicate whether a substance is
acidic or alkaline, but not the degree of acidity. pH indicator test papers
are also dipped into the solution, but the resulting color is matched to a
color standard to indicate
the pH of the sample.
Liquid pH indicators can
also be used to determine
pH. When the indicator is
added to a solution, the pH
of the solution causes the
indicator to change color,
which is matched to a
color standard to
determine the pH. The
most sophisticated method
of pH analysis is a pH
meter. When the pH
electrode is immersed in a
sample, the electrode and
meter combine to give a
pH reading which can be
read directly from the
meter.
4
Fig. 1 The approximate pH values of some
common substances.
