LaMotte AG-104 Salt Water Aquarium User Manual
Page 5
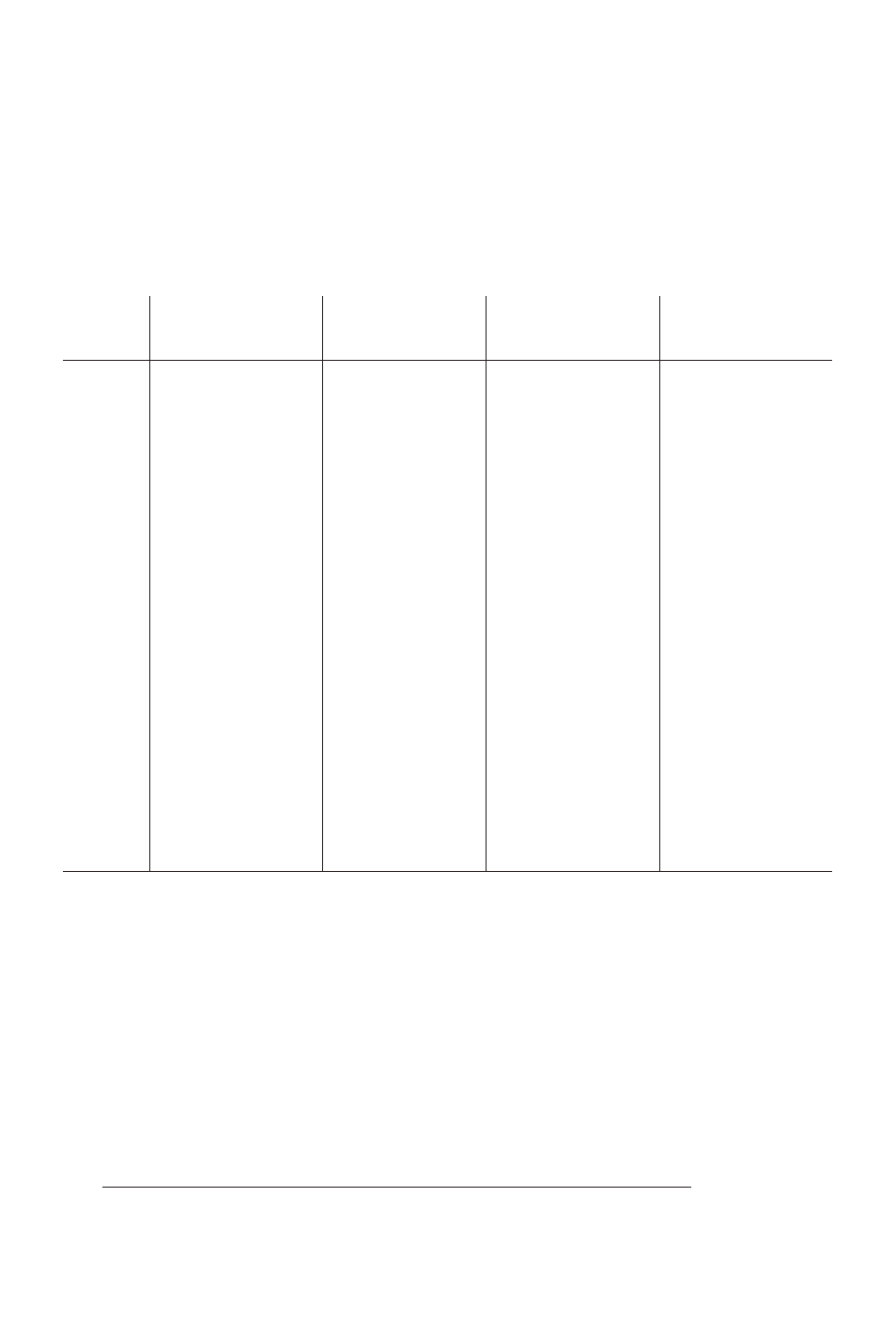
Consult the table to find the percentage that corresponds to the
temperature, pH, and salinity of the sample.
2. To express the test result as ppm Unionized Ammonia Nitrogen
(NH
3
–N), multiply the total ammonia-nitrogen test result by the
percentage from the table.
3. To express the test result as ppm Ionized Ammonia Nitrogen
(NH
4
+
–N), subtract the unionized ammonia-nitrogen determined in
Step 2 from the total ammonia-nitrogen.
10°C
15°C
20°C
25°C
pH
FW
1
SW
2
FW
SW
FW
SW
FW
SW
7.0
0.19
0.27
0.40
0.55
7.1
0.23
0.34
0.50
0.70
7.2
0.29
0.43
0.63
0.88
7.3
0.37
0.54
0.79
1.10
7.4
0.47
0.68
0.99
1.38
7.5
0.59
0.459
0.85
0.665 1.24
0.963
1.73
1.39
7.6
0.74
0.577
1.07
0.836 1.56
1.21
2.17
1.75
7.7
0.92
0.726
1.35
1.05
1.96
1.52
2.72
2.19
7.8
1.16
0.912
1.69
1.32
2.45
1.90
3.39
2.74
7.9
1.46
1.15
2.12
1.66
3.06
2.39
4.24
3.43
8.0
1.83
1.44
2.65
2.07
3.83
2.98
5.28
4.28
8.1
2.29
1.80
3.32
2.60
4.77
3.73
6.55
5.32
8.2
2.86
2.26
4.14
3.25
5.94
4.65
8.11
6.61
8.3
3.58
2.83
5.16
4.06
7.36
5.78
10.00
8.18
8.4
4.46
3.54
6.41
5.05
9.09
7.17
12.27
10.10
8.5
5.55
4.41
7.98
6.28
11.18
8.87
14.97
12.40
1
Freshwater data from Trussel (1972).
2
Seawater values from Bower and Bidwell (1978). Salinity for Seawater values = 34% at an
ionic strength of 0.701m.
EXAMPLE:
A fresh water sample sample at 20°C has a pH of 8.5 and the test
result is 1.0 ppm as Total Ammonia Nitrogen.
1. The percentage from the table is 11.18% (or 0.1118).
2. 1.0 ppm Total Ammonia Nitrogen x 0.1118 = 0.1118 ppm
Unionized Ammonia Nitrogen.
3. Total Ammonia-Nitrogen
1.0000 ppm
Unionized Ammonia-Nitrogen
- 0.1118 ppm
Ioinized Ammonia-Nitrogen
= 0.8882 ppm
5
