1 hydrometer readings, 2 correction for temperature, 3 correction for electrolyte level – Exide Technologies Section 93.10 User Manual
Page 13: 4 specific gravity range, 0 cell voltage variation
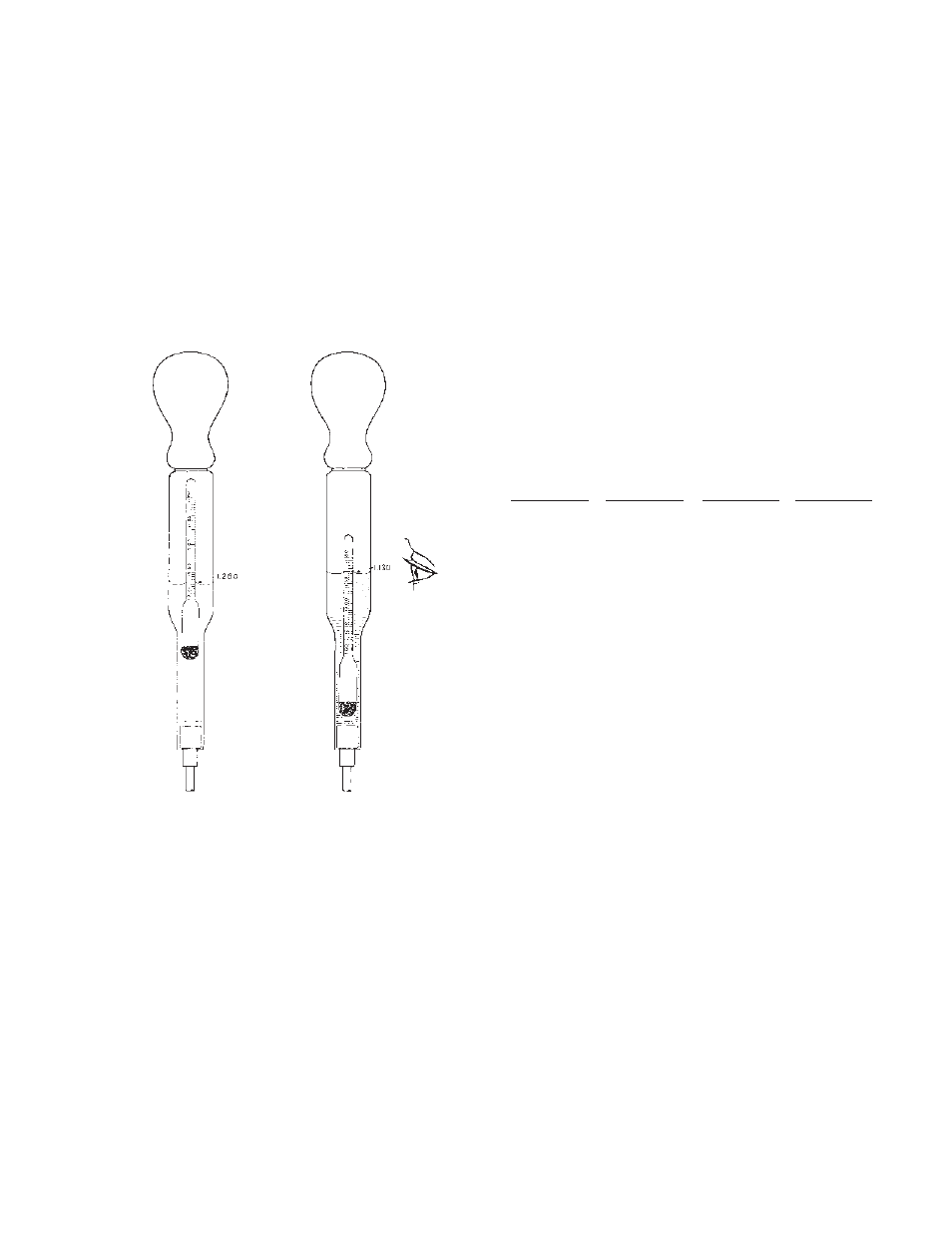
11.1 Hydrometer Readings
Specific gravity is used in determining a cell’s state of
charge. It decreases as the cell discharges and increases
as the cell is charged; reaching its original value when the
cell is fully charged. Specific gravity is expressed to the third
decimal place (1.215) and is measured by a hydrometer
float enclosed in a glass barrel/rubber bulb syringe. Draw
sufficient electrolyte into the barrels holding the syringe ver-
tical and with no hand pressure on bulb; so that float is freely
floating without touching sides or top of syringe.
The gravity is read on the hydrometer scale at the flat sur-
face of the electrolyte. (See Figure 5).
Clean the hydrometer glass barrel and float with soap and
water as required for ease of reading and float accuracy.
When recharging a lead-calcium cell, the specific gravity
reading lags behind the ampere hour input due mainly to the
very low end of charge currents. Mixing of the electrolyte is
slow due to the small amount of gas generated; so the grav-
ity readings do not reflect the actual state of charge. A simi-
lar condition exists after water additions. Therefore, mean-
ingful gravity readings can only be obtained at the top of the
cell after an equalizing charge or after six weeks on float.
For this reason, most GNB lead-calcium cells have elec-
trolyte withdrawal tubes to permit sampling of the electrolyte
at a point one third down from the top of the plates. A long
rubber tip on the hydrometer is inserted into the tube to pro-
vide an average value of cell specific gravity and a more
accurate indication on the state of the charge.
When taking a hydrometer reading, the base of the hydro-
meter syringe should be pressed firmly against the tube
opening to prevent back splash of electrolyte. Fill and empty
the hydrometer at least once in each cell before reading.
This will give a more accurate reading of the average elec-
trolyte density.
Never inter-mix usage of hydrometers on lead-antimony or
lead-calcium types as cell contamination will result. Assign
hydrometers for exclusive use on one type only.
11.2 Correction for Temperature
When taking specific gravity readings, corrections must be
made for variations in temperature of the electrolyte. For
each 3°F (1.67°C) in temperature of the electrolyte above
77°F (25°C) add one point (.001) in specific gravity to the
observed hydrometer readings; and for each 3°F (1.67°C) in
temperature below 77°F (25°C) subtract one (.001) in spe-
cific gravity from the observed hydrometer reading.
Example:
Reading
Hydrometer
Cell
Corrected to
Reading
Temperature
Correction
77°F (25°C)
1.213 sp. gr
68°F (20°C)
-.003 points=
1.210 sp. gr.
1.207 sp. gr.
86°F (30°C)
+.003 points=
1.210 sp. gr.
1.204 sp. gr.
95°F (35°C)
+.006 points=
1.210 sp. gr.
11.3 Correction for Electrolyte Level
The loss of water from the electrolyte due to evaporation as
well as conversion of the water to hydrogen and oxygen by
charging current also effects the specific gravity value. For
example: A fully charged cell with a correct high level at
77°F (25°C) will have a nominal specific gravity of 1.215.
When the electrolyte level has been reduced from evapora-
tion and charging by 1/4”, the specific gravity will be approx-
imately 6 points (.006) higher or 1.221@ 77°F (25°C).
Therefore when taking hydrometer readings, the electrolyte
level referenced to the high level line should be recorded for
proper evaluation of the specific gravity value. This applies
when taking a pilot cell reading or for 10% of the cells when
taking a quarterly set of readings.
11.4 Specific Gravity Range
GNB
stationary batteries are furnished with a nominal fully
charged specific gravity of 1.215@ 77°F (25°C).
For special applications, nominal specific gravity such as
1.250 or 1.300 @ 77°F (25°C) may be used.
The specific gravity may range± .010 points within a battery
for any of the nominal values @ 77°F (25°C) with the elec-
trolyte level at the high level line and still be considered sat-
isfactory.
SECTION 12
12.0 Cell Voltage Variation
The tabulation on the following page indicates the normal cell
voltage variation that may exist with the battery on float and
no greater than a 5°F (2.78°C) variation in cell temperature.
10
Figure 5
