Altitude evaluation, Ontour – Bayer HealthCare Ascensia Contour Blood Glucose Monitoring System User Manual
Page 36
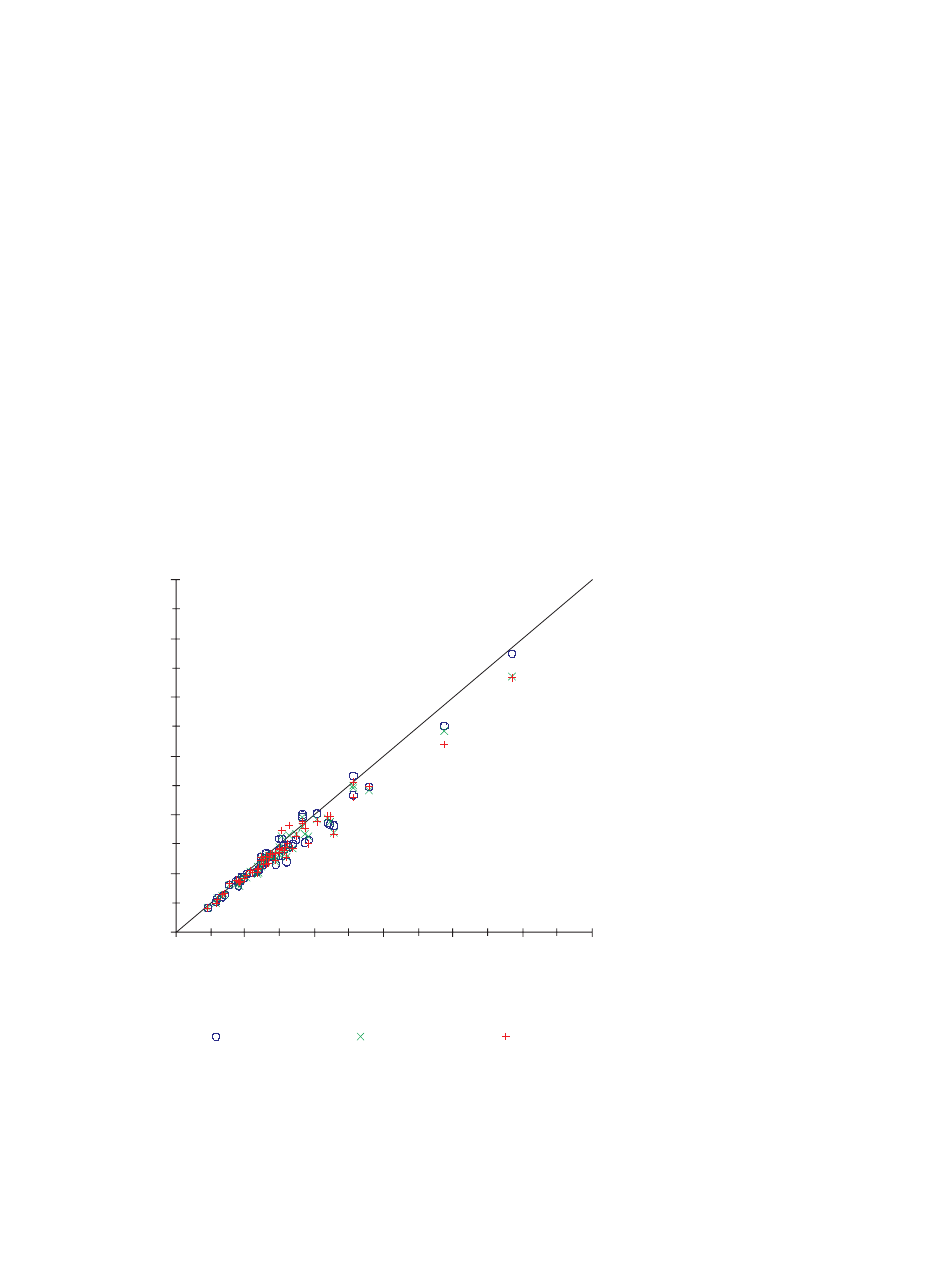
Altitude Evaluation
Performance was evaluated at an altitude of 10,000 ft.
Fifty-six samples were obtained from 54 different subjects
with diabetes. Three reagent lots were used with the
Ascensia C
ONTOUR
System to measure each subject’s capillary
blood glucose. After performing the Meter assays, additional
capillary blood was collected for a comparative blood glucose
(Yellow Springs Instruments Analyzer) and hematocrit
determination. The glucose range of the samples was 45 to
485 mg/dL, with an average concentration of 151 mg/dL. The
hematocrit range was 38 to 60%, with a mean of 48%.
32
Plasma Comparative Method Glucose Result (mg/dL)
Ascensia
C
ONTOUR
Result (mg/dL)
0
100
200
300
400
500
600
0
100
200
300
400
500
600
Lot 22B
Lot 22C
Lot 23B
y=x
