Merit Medical Centros FLO IFU User Manual
Page 7
CATHETER PERFORMANCE:
CAUTION: Always review hospital or unit protocol, potential
complications and their treatment, warnings, and precautions prior
to undertaking any type of mechanical or chemical intervention in
response to catheter performance problems.
WARNING: Only a physician familiar with the appropriate techniques
should attempt the procedures within this IFU.
INSUFFICIENT FLOWS:
The following may cause insufficient blood flows for dialysis:
• Kinked catheter, usually in subcutaneous tract.
Occluded arterial and/or venous lumen due to clotting or fibrin
sheath around the catheter.
Solutions include:
• Chemical intervention utilizing a thrombolytic agent.
• Vigorous flushing of the catheter with saline.
MANAGEMENT OF ONE-WAY OBSTRUCTIONS:
One-way obstructions exist when a lumen can be flushed easily but
blood cannot be aspirated. This is usually caused by tip malposition
but is sometimes due to a clot or fibrin sheath. One of the following
adjustments may resolve the obstruction:
• Reposition catheter
• Reposition patient
• Have patient cough
• Provided there is no resistance, flush the catheter vigorously with
sterile normal saline to try to open or move the tip.
• Other interventions as above.
INFECTION:
There is a risk of infection related to use of the catheter.
CAUTION: Due to the risk of exposure to Human Immunodeficiency
Virus (HIV) or other blood borne pathogens, health care professionals
should always use universal blood and body fluid precautions in the
care of all patients.
• Sterile technique should always be strictly adhered to.
• Clinically recognized infection at a catheter exit site should be
treated promptly with the appropriate antibiotic therapy.
• If a fever occurs in a patient with a catheter in place, take cultures
from a peripheral site (or dialysis line) and from one catheter
lumen. Culture catheter exit site if purulence is seen. Implement
the appropriate antibiotic therapy and consider removing catheter
if there are signs of sepsis. Wait 48 hours before catheter
replacement. Insertion should be made on opposite side of
original catheter exit site, if possible.
OVER-THE-WIRE TECHNIQUE:
CAUTION: Over the wire placement should only be performed by
a physician familiar with this technique. The peelaway sheath is not
used with this placement.
1. Advance guidewire with forward motion through the introducer
needle into the target vein.
2. Remove needle leaving the guidewire in the target vein.
3. Thread dilator(s) over guidewire into the vein (a slight twisting
motion may be used). Remove dilator(s) when vein is sufficiently
dilated, leaving the guidewire in place. Apply pressure to incision
when dilators are removed.
4. Thread the proximal end of the guidewire through the distal tip
of the venous lumen and the slit as indicated by the
+
sign
on the
catheter.
5. Thread the guidewire through the distal tip of the arterial lumen
and through the catheter lumen until the proximal end of the guide-
wire exits the arterial luer on the extension set.
6. Advance the catheter until the distal tip of the arterial lumen is
within the primary incision.
NOTE: Ensure blood is coming out of the arterial lumen while advanc-
ing the catheter.
CAUTION: DO NOT advance guidewire with catheter into vein.
Cardiac arrhythmia may result if guidewire is allowed to pass into the
right atrium. The guidewire should be held securely during catheter
placement.
7. Gently remove the guidewire, leaving catheter in place.
8. Make any adjustments to catheter under fluoroscopy.
9. Continue with step number 7 under “Dialysis Catheter Placement”
section.
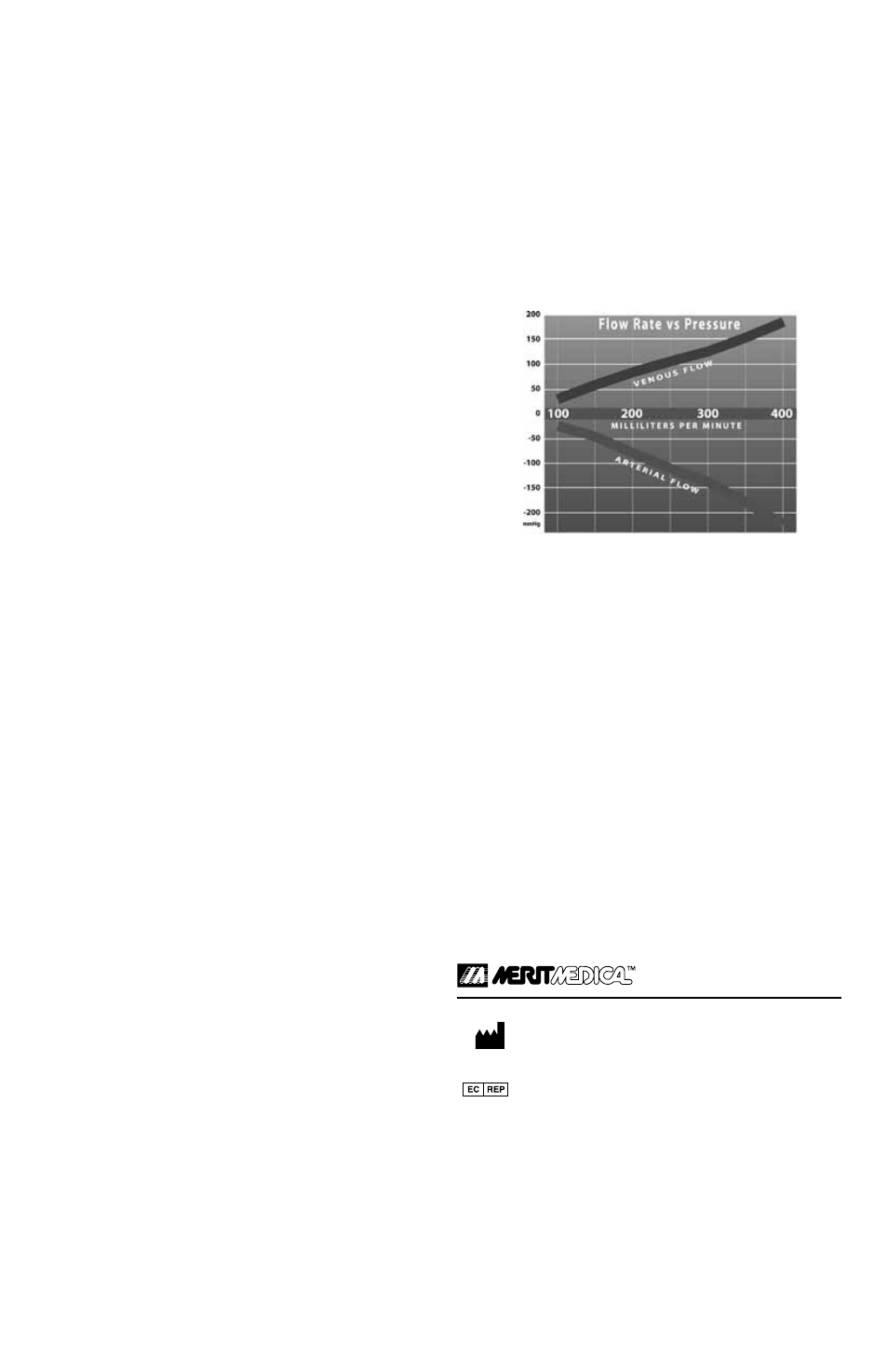
Flow vs. Pressure Data
NOTE: Flow testing represents optimum bench test laboratory condi-
tions. 23cm tip-cuff catheter samples were used in a simulated blood
and anatomical model.
REFERENCES: Lebanc, M., Bosc, J., Paganini, E., & Canaud, B., Central
Venous Dialysis Catheter Dysfunction: Advances in Renal Replace-
ment Therapy. 1997:4(4):377-389. Hirsch, D., Bergen, P., & Jindal, K.,
Polyurethane Catheters for Long-Term Hemodialysis Access: Artificial
Organs. 1997:21(5):349-354.
Centros® & CentrosFLO™ are registered trademarks of Merit Medical
Systems, Inc.
The third party trademark identified above are the property of their
respective trademark owners.
Catheter kit contents will include (1) Hemodialysis Catheter and
accessories. For exact kit contents refer to the product label.
Manufacturer: www.merit.com
Merit Medical Systems, Inc. South Jordan, Utah 84095
U.S.A. 1-801-253-1600 U.S.A. Customer Service 1-800-356-3748
Authorized Representative:
Merit Medical Ireland Ltd,
Parkmore Business Park West, Galway, Ireland
European Customer Service by Country:
Belgium 0800 72906; France 0800 916030; Germany 0800 1820871;
Ireland 091 703700; Neth. 0800 0228184; U.K. 0800 973115 I
ID 042312 402490001/C
