Thermo Fisher Scientific Ion Selective Electrodes Silver User Manual
Page 9
Instruction Manual
Silver/Silver Sulfide Electrode
9
5.
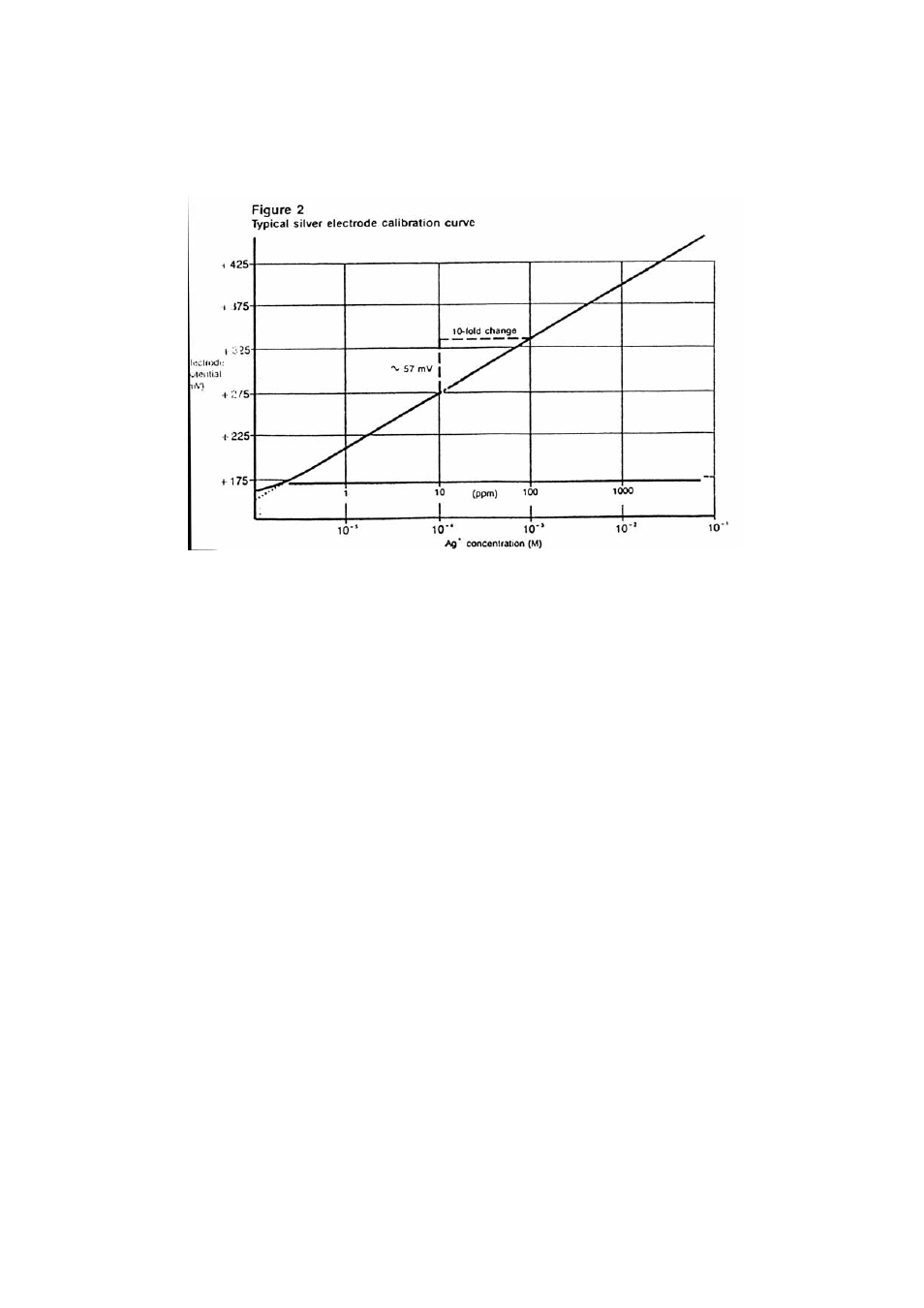
Using the semi-logarithmic graph paper, plot the mV reading (linear axis) against
concentration (log axis). Extrapolate the curve down to about 2.0X10-
6
M or 0.2 ppm. A
typical calibration curve can be found in Figure 2.
A calibration curve is constructed on semi-logarithmic paper when using a
pH/mV meter in the millivolt mode. The measured electrode potential in
mV (linear axis) is plotted against the standard concentration (log axis). In
the linear region of the curve, only three standards are necessary to
determine a calibration curve. In the non-linear region, additional points
must be measured. The direct measurement procedures given are for the
linear portion of the curve. The non-linear portion of the curve requires the
use of low level procedures.
6.
To a clean, dry 150 ml beaker, add 100 ml of sample and 2 ml of ISA. Place the beaker on
the magnetic stirrer and begin stirring. Place the electrode tips in the solution. When the
reading has stabilized, record the mV reading. Determine the concentration directly from
the calibration curve.
7.
The calibration should be checked every two hours. Assuming no change in ambient
temperature, place the electrode tips in the mid-range standard. After the reading has
stabilized, compare it to the original reading recorded in Step 3 above. A reading differing
by more than 0.5 mV or a change of ambient temperature will necessitate the repetition of
Steps 2-5 above. A new calibration curve should be prepared daily.
Direct Measurement of Silver (using an ion meter)
1.
By serial dilution of the 0.1M or 1,000 ppm silver standard, prepare two silver standards
whose concentration is near the expected sample concentration. Measure out 100 ml of
each standard into individual 150 ml beakers and add 2 ml of ISA to each.
