Thermo Fisher Scientific Ion Selective Electrodes Silver User Manual
Page 14
Silver/Silver Sulfide Electrode
Instruction Manual
14
Titration of Sulfide
The minimum sulfide sample concentration for this method is 1.0X10-
5
M. The titrant to be used is
a lead perchlorate standard solution.
1.
Using Eutech Lead Perchlorate Solution, 0.1M, Code No. EC-SCS-PB1-BT, prepare a lead
titrant that is about 10-20 times as concentrated as the expected sample concentration by
dilution.
2.
Dilute 25 ml of the sample with 25 ml of SAOB in a 150 ml beaker. Place the beaker on
the magnetic stirrer and begin stirring. Lower the tips of the electrodes into the solution.
3.
Using a 10 ml burette, add titrant in 0.5-1.0 ml increments. Record the mV reading against
the volume of titrant added. As the mV potential change increases, add smaller increments,
down to 0.1-0.2 ml increments. Continue to add titrant and record the mV potential against
the volume until little change is noted in the mV reading even when adding 0.5-1.0 ml
increments.
4.
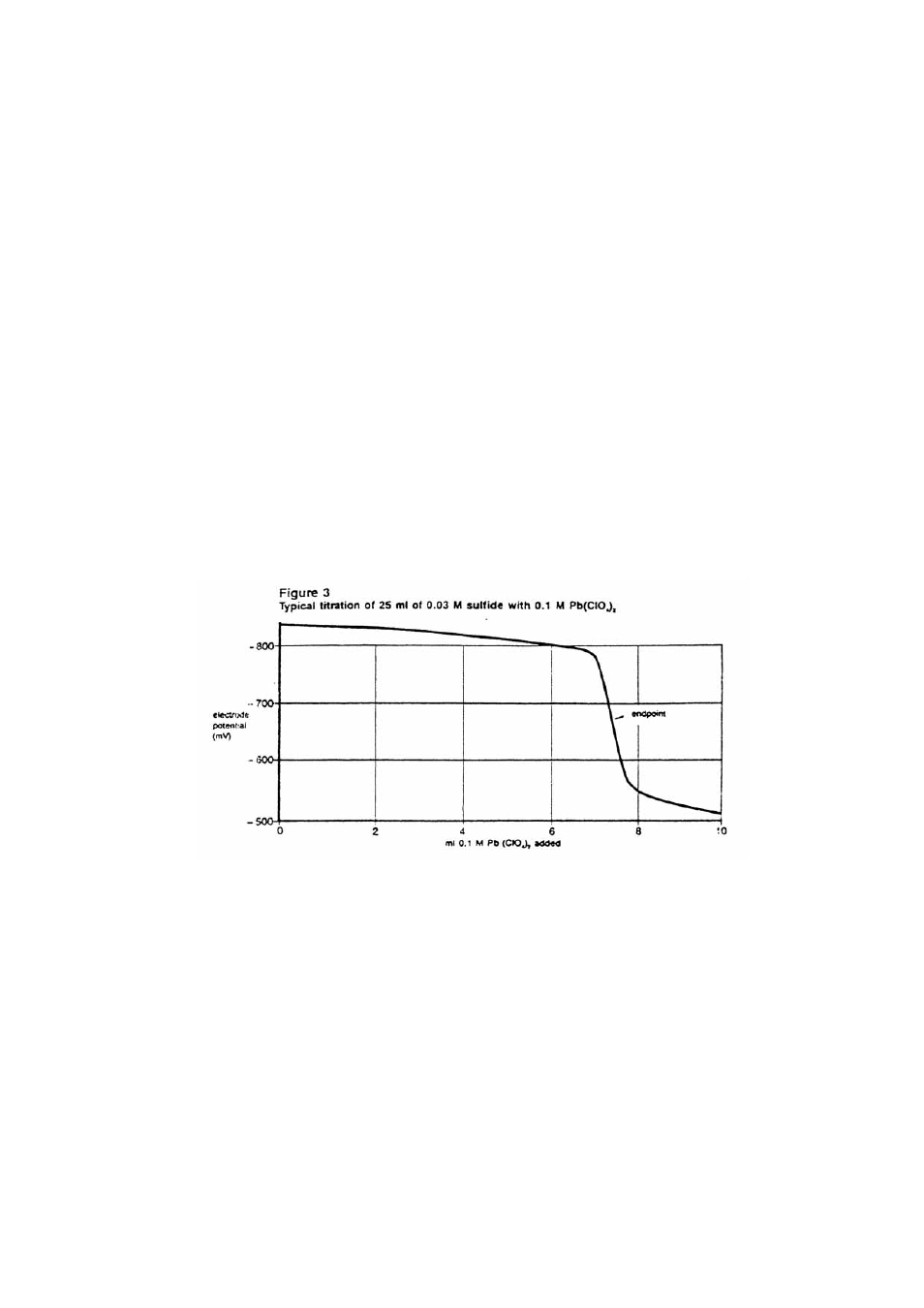
Using linear graph paper, plot the mV readings (y-axis) against the volume (x-axis). The
end point is determined at the steepest slope on the titration curve as illustrated in Figure 3.
5.
The sample concentration, Cs, is calculated before the dilution with SAOB, as follows:
Cs = (Vt/Vs) Ct
where
Vs = sample volume before dilution (25 ml)
Vt = titrant volume at endpoint
Ct = titrant concentration (M)
Titration of Silver
Eutech Silver/Sulfide Ion Electrode is a highly sensitive endpoint detector for silver titration with a
halide standard solution. It can also be used as an indicator for halide titration with a silver standard
solution.
