Thermo Fisher Scientific Ion Selective Electrodes Calcium User Manual
Page 13
Instruction Manual
Calcium Electrode
13
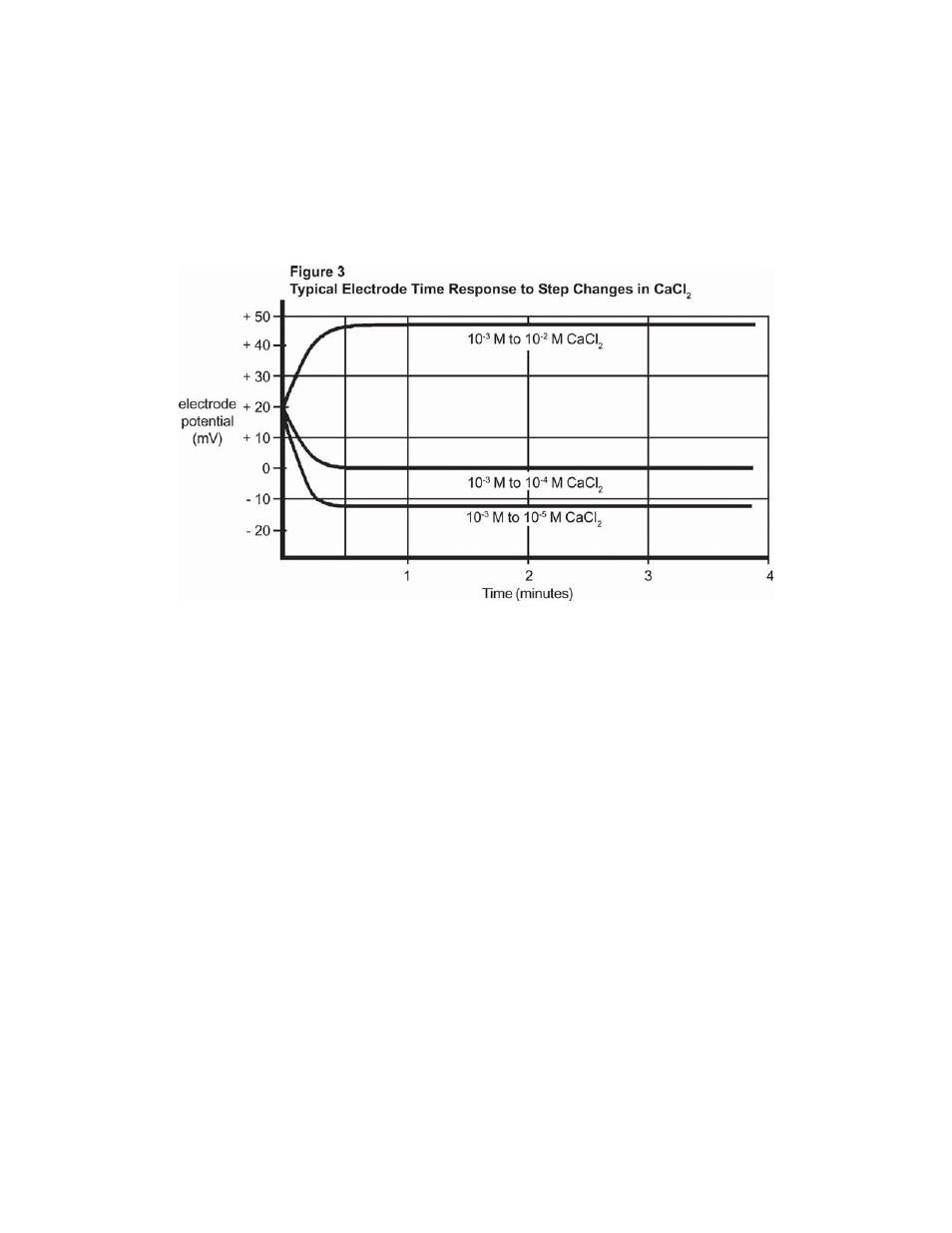
Electrode Response
Plotting the mV potential against the calcium concentration on semi-logarithmic paper results in a
straight line with a slope of about 27 mV per decade (Refer to Figure 1).
The time needed to reach 99% of the stable electrode potential reading, the electrode response time,
varies from one minute or less for calcium concentration above 1.0x10-4M to several minutes near
the detection limit. (Refer to Figure 3.)
Limits of Detection
The upper limit of detection in pure calcium chloride solutions is 1M. In the presence of other ions,
the upper limit of detection is above 1.0x10
-1
M, but the possibility of a liquid junction potential
developing at the reference electrode and the "salt extraction effect" are two limiting factors. Some
salts may infuse into the electrode membrane at high salt concentrations causing deviation from
theoretical response. Calibrate the electrode at four or five intermediate points, or dilute the sample,
to measure samples between 1.0x10
-1
M and 1M.
The lower limit of detection is influenced by the slight water solubility of the ion exchanger used in
the sensing portion of the electrode. Refer to Figure 1 for a comparison of the theoretical response
to actual response at low levels of calcium chloride.
pH Effects
The operating range of the calcium electrode is from pH 3 to pH 10. Use at other pH values can
adversely affect the membrane. Hydrogen ion interferes with measurements of very low levels of
calcium. Hydroxide ion will complex calcium ions.
Electrode Life
The calcium electrode will last six months in normal laboratory use. On-line measurement might
shorten operational lifetime to several months. In time, the response time will increase and the
calibration slope will decrease to the point calibration is difficult and electrode replacement is
required.
