Dissolved oxygen theory, Do solubility in water vs temperature – Thermo Fisher Scientific Eutech DO 6 Plus User Manual
Page 43
Instruction Manual
DO 6+
- 37 -
12. DISSOLVED OXYGEN THEORY
Dissolved Oxygen (DO) refers to the volume of oxygen that is contained in
water. There are two main sources of DO in water; atmosphere and
photosynthesis. Waves and tumbling water mix air into the water where oxygen
readily dissolves until saturation occurs. Oxygen is also produced by aquatic
plants and algae during photosynthesis.
The amount of DO that can be held by water depends on 3 factors:
1) TEMPERATURE:
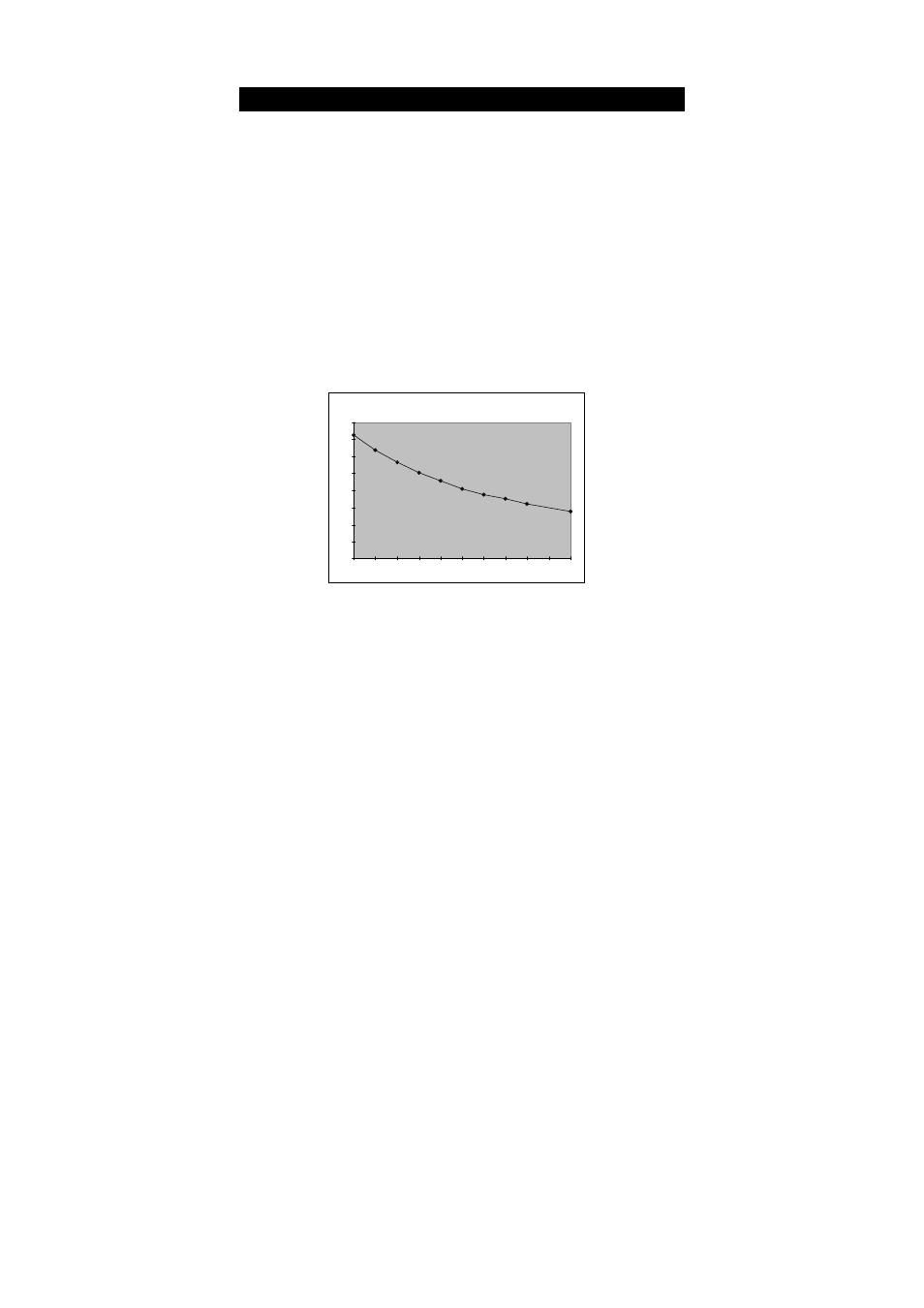
DO increases with decreasing temperature
(colder water holds more oxygen)
2) SALINITY:
DO increases with decreasing salinity
(freshwater holds more oxygen than saltwater does)
3) ATMOSPHERIC PRESSURE:
DO decreases with decreasing atmospheric pressure
(amount of DO absorbed in water decreases as altitude increases)
Solubility of oxygen in water contact with water saturated air at
standard atmospheric pressure
0
2
4
6
8
10
12
14
16
0
5
10
15
20
25
30
35
40
45
50
Temperature °C
S
o
lu
b
ili
ty
m
g
/L
DO Solubility in Water vs Temperature
