How does it work, What is a volatile organic compound (voc) – Ion Science MiniPID User Manual
Page 7
MINIPID 3PIN MANUAL
Ion Science Ltd
Page 7 of 27
Unrivalled Detection. www.ionscience.com
How does it work?
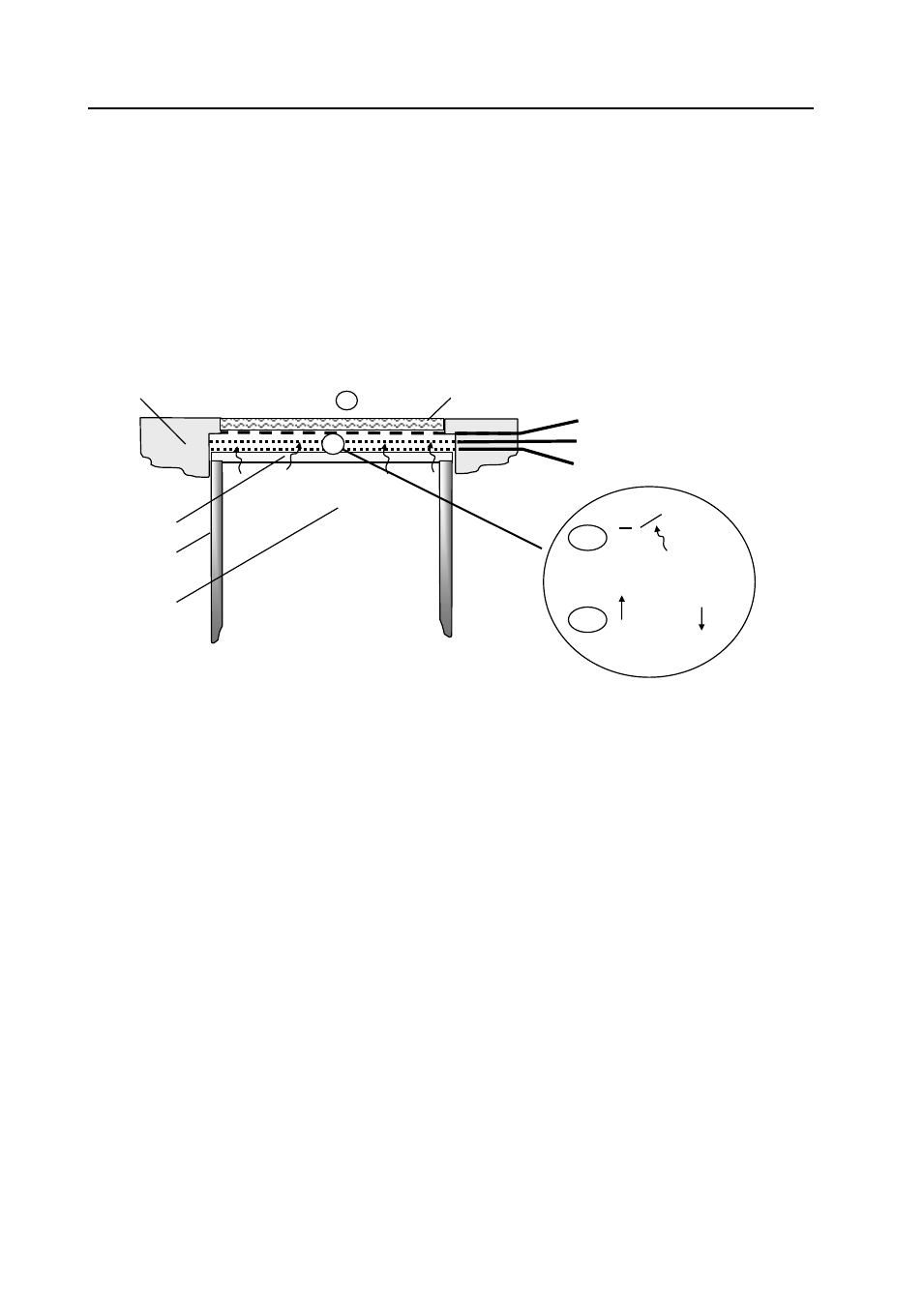
The Ion Science MiniPID measures volatile organic compounds (VOCs) in air by photoionisation detection
(PID), which is shown schematically below. Test gas (1) is presented to the membrane filter at the top of the
photoionisation cell and freely diffuses into and out of the underlying chamber formed by the filter, housing
walls, and a UV lamp window. The lamp emits photons (shown by arrows) of high energy UV light,
transmitted through the window. Photoionisation occurs in the chamber when a photon is adsorbed by the
molecule, generating two electrically charged ions, one positively charged, X
+
, and one negatively charged,
Y
-
(2a). An electric field, generated between the cathode and anode electrodes, attracts ions (2b). The
resulting current, which is proportional to the concentration of the VOC, is measured and used to determine
the gas concentration. The MiniPID includes a third fence electrode (patented) to ensure that the amplified
current does not include significant contributions due to other current sources such as water condensation on
the chamber walls.
What is a volatile organic compound (VOC)?
A volatile organic compound, or VOC, is a carbon-containing chemical, which is significantly or completely
vaporised at ambient temperatures.
What volatile organic compound (VOCs) are sensed by MiniPID?
Most VOC’s can be detected by MiniPID. Notable exceptions are low molecular weight hydrocarbons. Each
VOC has a characteristic threshold energy of light (photon energy) which, when directed at the VOC, causes
it to fragment into ions. This is called the Ionisation Potential, or IP. VOCs are ionised (and hence detected) if
light of photon energy greater than the IP interacts with the gas sample. The peak photon energy generated
in a detector depends on the PID lamp used: Krypton = 10.6 eV or Argon = 11.7 eV. Hence, the use of an
argon lamp leads to detection of the largest range of volatile compounds, while using a Xenon lamp can
increase selectivity. Lamps of a particular type do not typically vary in spectral fingerprint, so relative
responses to a particular gas, eg benzene, to a particular lamp, e.g. krypton, does not vary from lamp to
lamp. However, the intensity of lamps does vary to some extent, leading to a difference in absolute
response to the calibration gas.
Sufficient volatility of a compound is also essential for measurement by PID as with any other detector. A
fairly large molecule such as alpha pinene, (a constituent of turpentine), saturates in air at about 5000 ppm
at 20
o
C; this is the maximum concentration at which the compound will usually be detected. Some
compounds, for example, machine oils and agrochemicals - generate only a few ppm of vapour at ambient
temperatures; it is more difficult to detect these compounds in air.
‘MiniPID response factors’ Application
Note lists VOCs by their common name and their sensitivity to a Krypton lamp, the most common lamp and
the lamp supplied with the MiniPID PPM and MiniPID PPB.
To anode
Lamp gas, eg
krypton
Lamp
window
Test gas
Lamp
body
Cathode
Anode
Fence electrode
2
2b
2a
Y
X
X
+
Photon
Copyright Ion Science Ltd, 2007
1
To cathode
Y
-
