Hanna Instruments HI 83746 User Manual
Page 4
6
7
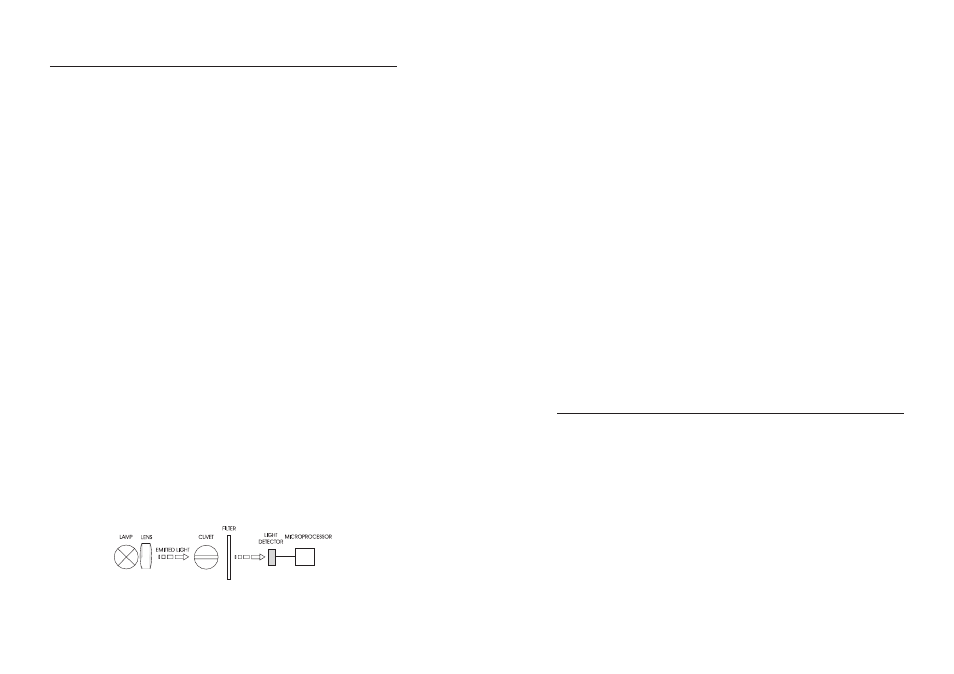
A microprocessor controlled special tungsten lamp emits radiation which is first optically conditioned
and beamed to the sample contained in the cuvet. The optical path is fixed by the diameter of the
cuvet. Then the light is spectrally filtered to a narrow spectral bandwidth, to obtain a light beam of
intensity
I
o
or
I.
The photoelectric cell collects the radiation
I
that is not absorbed by the sample and converts it
into an electric current, producing a potential in the mV range.
The microprocessor uses this potential to convert the incoming value into the desired measuring
unit and to display it on the LCD.
The measurement process is carried out in two phases: first the meter is zeroed and then the actual
measurement is performed.
The cuvet has a very important role because it is an optical element and thus requires particular
attention. It is important that both the measurement and the calibration (zeroing) cuvets are
optically identical to provide the same measurement conditions. Whenever possible use the same
cuvet for both. It is necessary that the surface of the cuvet is clean and not scratched. This to avoid
measurement interference due to unwanted reflection and absorption of light. It is recommended
not to touch the cuvet walls with hands.
Furthermore, in order to maintain the same conditions during the zeroing and the measuring
phases, it is necessary to close the cuvet to prevent any contamination.
degree Celsius
degree Fahrenheit
grams per liter. g/L is equivalent to ppt (part per thousand)
milliliter
microliter
Liquid Crystal Display
°C:
°F:
g/L:
mL:
µL:
LCD:
ABBREVIATIONS
PRINCIPLE OF OPERATION
Absorption of Light is a typical phenomenon of interaction between electromagnetic radiation and
matter. When a light beam crosses a substance, some of the radiation may be absorbed by atoms,
molecules or crystal lattices.
If pure absorption occurs, the fraction of light absorbed depends both on the optical path length
through the matter and on the
physical
-chemical characteristics of the substance according to the
Lambert-Beer Law:
-log
I
/
I
o
=
ε
λ
c d
or
A
=
ε
λ
c d
Where:
-log
I
/
I
o
=
Absorbance (A)
I
o
=
intensity of incident light beam
I
=
intensity of light beam after absorption
ε
λ
=
molar extinction coefficient at wavelength
λ
c
=
molar concentration of the substance
d
=
optical path through the substance
Therefore, the concentration "c" can be calculated from the absorbance of the substance as the
other factors are known.
Photometric chemical analysis is based on the possibility to develop an absorbing compound from
a specific chemical reaction between sample and reagents. Given that the absorption of a
compound strictly depends on the wavelength of the incident light beam, a narrow spectral
bandwidth should be selected as well as a proper central wavelength to optimize measurements.
The optical system of Hanna's HI 83000 series colorimeters is based on special subminiature
tungsten lamps and narrow-band interference filters to guarantee both high performance and
reliable results.
Block diagram (optical layout)
