Hanna Instruments HI 4012 User Manual
Page 15
15
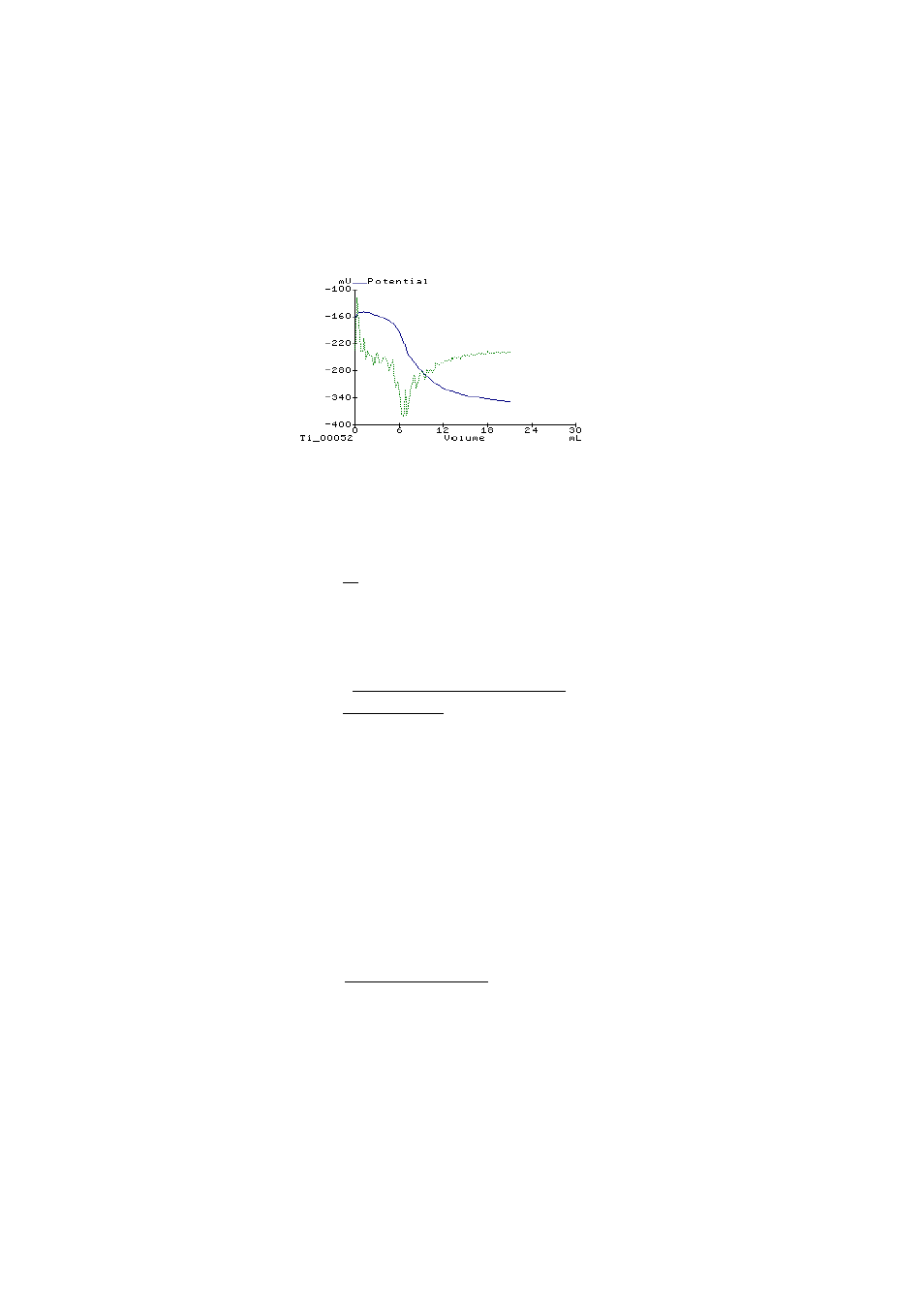
Plot generated on Hanna HI 901 Titrator during
Plot generated on Hanna HI 901 Titrator during
Plot generated on Hanna HI 901 Titrator during
Plot generated on Hanna HI 901 Titrator during
Plot generated on Hanna HI 901 Titrator during
automated lead titration using HI 4112 electrode.
automated lead titration using HI 4112 electrode.
automated lead titration using HI 4112 electrode.
automated lead titration using HI 4112 electrode.
automated lead titration using HI 4112 electrode.
Notes:
•
Use of the methanol/formalin antioxidant is
advised to increase sensitivity.
•
pH adjustment of the sample to 4-7 pH is advised to
favor the Pb-EDTA formation constant.
XIV. S
XIV. S
XIV. S
XIV. S
XIV. Storage and Care of the HI 4012 and
torage and Care of the HI 4012 and
torage and Care of the HI 4012 and
torage and Care of the HI 4012 and
torage and Care of the HI 4012 and
HI 4112 sensors
HI 4112 sensors
HI 4112 sensors
HI 4112 sensors
HI 4112 sensors
The HI 4012 and HI 4112 sensor can be stored in very
dilute standards (<10
-3
M) for short periods of time.
The HI4012 should be stored dry with it’s protective cap
on for longer periods. For longer term storage of the
model HI 4112 combination electrode ;Drain and wash
of salts with distilled or deionized water. Unscrew the
upper cap and move outer sleeve up cable. Wrap the
ceramic junction on the inner stem with Parafilm
® or
other sealing wrap. Place the protective cap provided
over the dried membrane and store in original storage
box provided with electrode.
XV
XV
XV
XV
XV.
.
.
.
. Conversion T
Conversion T
Conversion T
Conversion T
Conversion Tables For Pb
ables For Pb
ables For Pb
ables For Pb
ables For Pb
2+
2+
2+
2+
2+
Multiply
Multiply
Multiply
Multiply
Multiply
Moles/L (M) to ppm (mg/L)
2.072 x 10
5
ppm (mg/L) to M (moles/L)
4.826 x 10
-6
XIII.
XIII.
XIII.
XIII.
XIII.pH
pH
pH
pH
pH
HI 4112 and HI 4012 electrodes may be used in solutions
with pH values between 4 and 6. Samples that fall beyond
this range should be adjusted with perchloric acid or sodium
hydroxide.
