Bio-Rad Bio-Rex® Reactor Grade Resins User Manual
Page 6
Note: Formamide interferes with the color change of
the dye, but will not affect the deionization capacity
of the resin.
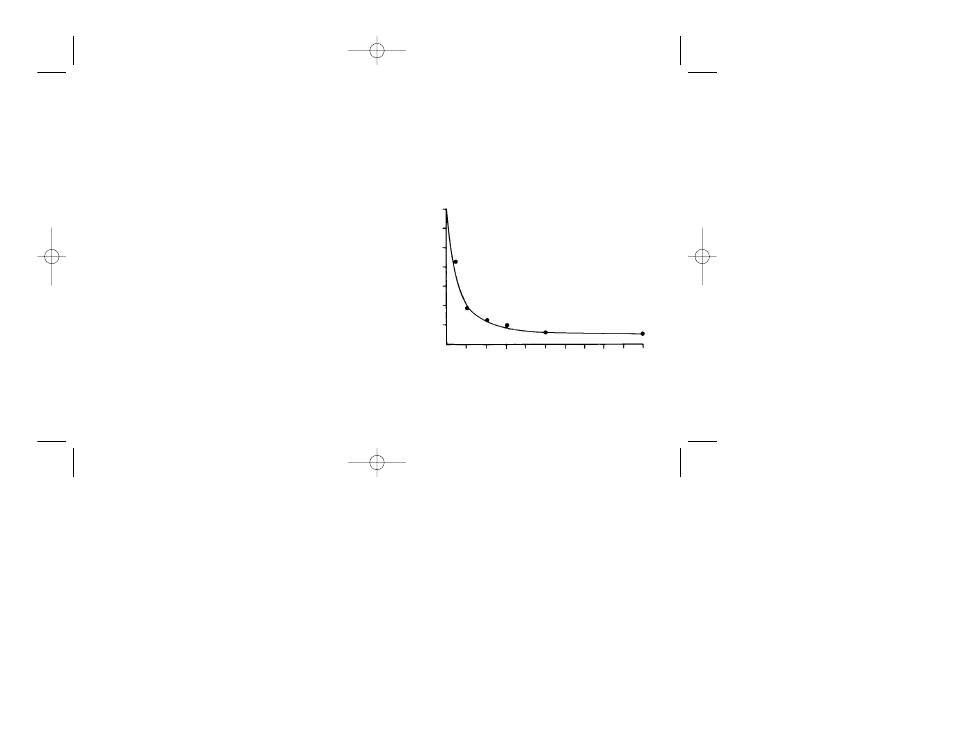
Batch vs. Column Deionization of Urea
Table 3 compares deionizing urea both by the batch
and the column technique. The decrease in conductivity
using the batch method is plotted in Figure 1.
Fig. 1. Decrease in conductivity using batch method.
9
formamide, glyoxal, or PEG, although the batch tech-
nique is much more common.
Batch Deionization of Formamide, Acrylamide,
and Glyoxal
This procedure was originally described by Maniatis,
et al. [Molecular Cloning: A Laboratory Manual, Cold
Spring Harbor Lab (1982)] for deionization of form-
amide, and can be used for any nonionic reagent such as
acrylamide, glyoxal, urea, or water.
1.
Weigh 5 grams of resin for every 100 ml of for-
mamide or acrylamide solution to be deionized. For
glyoxal, use 1 gram of resin per ml of glyoxal. This
quantity of resin will be sufficient for any concentra-
tion of these solutions.
2.
Wash resin briefly with the solution to be deionized,
using about 1 ml solution per ml of resin. Discard the
solution.
3.
Add resin to sample and stir for 1 hour. Check pH
with pH paper to insure deionization is complete.
Repeat Step 3 using new resin if necessary.
4.
Filter or decant sample from resin.
8
Conditions
Resin:
AG 501-X8 resin, 5 g
Sample:
100 ml 6 M urea
1
5
10
Hours
70
10
Conductivity µ
mhos/cm
LIT205B 6/17/98 12:15 PM Page 8
