Merit Medical QuadraSphere Microspheres User Manual
Page 2
2
3
4
5
• Presence or suspicion of vasospasm
• Presence or likely onset of haemorrhage
• Presence of severe atheromatous disease
• Feeding arteries too small to accept the selected
QuadraSphere Microspheres
• Presence of collateral vessel pathways potentially
endangering normal territories during embolization
• High flow arteriovenous shunts or fistulae with luminal
diameter greater than the selected size of QuadraSphere
Microspheres
• Vascular resistance peripheral to the feeding arteries
precluding passage of QuadraSphere Microspheres into the
lesion
• Presence of arteries supplying the lesion not large enough
to accept QuadraSphere Microspheres
• Do not use in pulmonary vasculature, coronary and
central nervous system vasculature
• Known sensitivity to poly vinyl alcohol-co-sodium
acrylate
WARNINGS:
• QuadraSphere Microspheres size must be chosen after
consideration
of
the
arteriovenous
angiographic
appearance. QuadraSphere Microspheres size should be
selected to prevent passage from any artery to vein.
• Some of the QuadraSphere Microspheres may be slightly
outside of the range, so the physician should be sure to
carefully select the size of QuadraSphere Microspheres
according to the size of the target vessels at the desired
level of occlusion in the vasculature and after consideration
of the arteriovenous angiographic appearance.
• Because
of
the
significant
complications
of
misembolization, extreme caution should be used for any
procedures
involving
the
extracranial
circulation
encompassing the head and neck, and the physician should
carefully weigh the potential benefits of using embolization
against the risks and potential complications of the
procedure.
These complications can include blindness,
hearing loss, loss of smell, paralysis, and death.
• Serious radiation induced skin injury may occur to the
patient due to long periods of fluoroscopic exposure, large
patient, angled x-ray projections and multiple image
recording runs or radiographs. Refer to your facility’s
clinical protocol to ensure the proper radiation dose is
applied for each specific type of procedure performed.
• Onset of radiation injury to the patient may be delayed.
Patients should be counselled on potential radiation effects,
what to look for and who to contact if symptoms occur.
• QuadraSphere Microspheres MUST NOT be reconstituted
in sterile water for injection. Reconstitution in sterile water
results in extensive swelling that renders the injection of
QuadraSphere Microspheres very difficult or may prevent
injection.
• Do not reconstitute QuadraSphere Microspheres with
Lipiodol / Ethiodol.
• Pay
careful
attention
for
signs
of
mistargeted
embolization. During injection carefully monitor patient vital
signs to include SaO
2
(e.g. hypoxia, CNS changes). Consider
terminating the procedure, investigating for possible
shunting, or increasing Microspheres size if any signs of
mistargeting occur or patient symptoms develop.
• Consider upsizing the Microspheres if angiographic
evidence of embolization does not quickly appear evident
during injection of the Microspheres.
Warnings about use of small microspheres:
• Careful consideration should be given whenever use is
contemplated of embolic agents that are smaller in
diameter than the resolution capability of your imaging
equipment. The presence of arteriovenous anastomoses,
branch vessels leading away from the target area or
emergent vessels not evident prior to embolization can lead
to mistargeted embolization and severe complications.
• Microspheres smaller than 100 microns will generally
migrate distal to anastomotic feeders and therefore are more
likely to terminate circulation to distal tissue. Greater potential
of ischemic injury results from use of smaller sized
microspheres
and consideration must be given to the
consequence of this injury prior to embolization. The potential
consequences include swelling, necrosis, paralysis, abscess
and/or stronger post-embolization syndrome.
• Post embolization swelling may result in ischemia to
tissue adjacent to target area. Care must be given to avoid
ischemia of intolerant, non targeted tissue such as nervous
tissue.
PRECAUTIONS:
QuadraSphere Microspheres must only be used by
physicians trained in vascular embolization procedures. The
size and quantity of microspheres must be carefully
selected according to the lesion to be treated and the
potential presence of shunts. Only the physician can decide
the most appropriate time to stop the injection of
QuadraSphere Microspheres.
Do not use if the vial, cap, or pouch appear damaged.
For single patient use only - Contents supplied sterile -
Never reuse, reprocess, or resterilize the contents of a vial
that has been opened. Reusing, reprocessing or resterilizing
may compromise the structural integrity of the device and
or lead to device failure, which in turn may result in patient
injury, illness or death. Reusing, reprocessing or
resterilizing may also create a risk of contamination of the
device and or cause patient infection or cross infection
including, but not limited to, the transmission of infectious
disease(s) from one patient to another. Contamination of the
device may lead to injury, illness or death of the patient. All
procedures must be performed according to accepted
aseptic technique.
QuadraSphere Microspheres MUST NOT be used in their
original dry state. They must be reconstituted before use.
QuadraSphere Microspheres swell in aqueous solution. The
magnitude of swelling depends on the ionic concentration
of the solution. The microspheres swell to approximately
four times their diameter in 0.9% NaCl aqueous solution
and non-ionic contrast media, as compared to their initial
dry
diameter.
QuadraSphere
Microspheres
are
compressible and can be injected easily through
microcatheters. However, injection of the QuadraSphere
Microspheres before they are fully expanded could result in
failure to reach the intended embolization target and
possible embolization of a larger tissue area.
Patients with known allergies to non-ionic contrast media
may require corticosteroids prior to embolization.
Additional evaluations or precautions may be necessary in
managing periprocedural care for patients with the
following conditions:
• Bleeding diathesis or hypercoagulative state
• Immunocompromise
POTENTIAL COMPLICATIONS:
Vascular
embolization
is
a
high-risk
procedure.
Complications may occur at any time during or after the
procedure, and may include, but are not limited to, the
following:
• Paralysis resulting from untargeted embolization or
ischemic injury from adjacent tissue oedema
• Undesirable reflux or passage of QuadraSphere
Microspheres into normal arteries adjacent to the targeted
lesion or through the lesion into other arteries or arterial
beds, such as the internal carotid artery, pulmonary, or
coronary circulation
• Pulmonary embolism due to arteriovenous shunting
• Ischemia at an undesired location, including ischemic
stroke, ischemic infarction (including myocardial infarction),
and tissue necrosis
• Capillary bed occlusion and tissue damage
• Vasospasm
• Recanalisation
• Blindness, hearing loss, and loss of smell
• Foreign body reactions necessitating medical intervention
• Infection necessitating medical intervention
• Complications related to catheterization (e.g. haematoma
at the site of entry, clot formation at the tip of the catheter
and subsequent dislodgement, and nerve and/or circulatory
injuries which may result in leg injury)
• Allergic reaction to medications (e.g. analgesics)
• Allergic reaction to non-ionic contrast media or embolic
material
• Vessel or lesion rupture and haemorrhage
• Death
• Additional information is found in the Warnings section
SWELLING BEHAVIOR:
QuadraSphere Microspheres swell during reconstitution
with NaCl 0.9% aqueous solution and non-ionic contrast
media. When hydrated in 100% NaCl 0.9% aqueous
solution or non-ionic contrast medium, or 50% non-ionic
contrast and 50% NaCl 0.9% aqueous solution,
QuadraSphere Microspheres swell approximately 4 times
their original dry diameter in approximately 10 minutes. For
example, QuadraSphere Microspheres with a diameter of
approximately 50-100 microns in their dry state will expand
to approximately 200-400 microns during reconstitution as
recommended below. Because of the inherent variability of
the swelling process, some of the QuadraSphere
Microspheres will be slightly outside of this range after
reconstitution, so the physician should be sure to carefully
select the size of QuadraSphere Microspheres according to
the size of the target vessels at the desired level of
occlusion in the vasculature and the nature of the aqueous
solution.
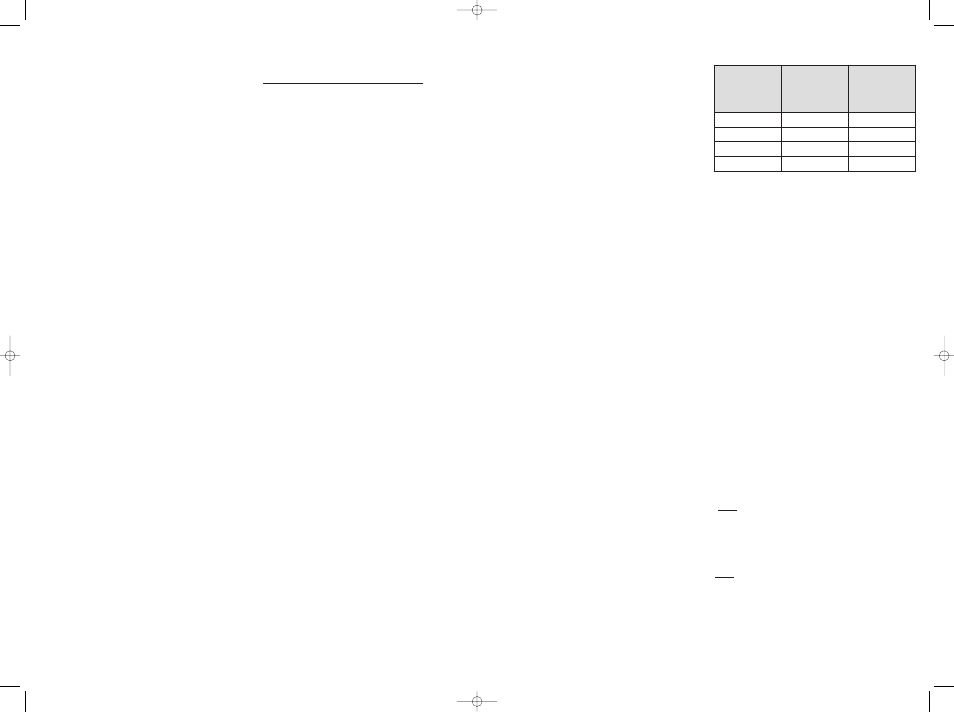
CATHETER COMPATIBILITY:
QuadraSphere Microspheres can be injected with
microcatheters with the following specifications:
INSTRUCTIONS:
QuadraSphere Microspheres must be reconstituted with
100% NaCl 0.9% aqueous solution or non-ionic contrast
medium, or 50% non-ionic contrast medium and 50% NaCl
0.9% aqueous solution.
• Carefully select the size of QuadraSphere Microspheres
according to the size of the target vessels at the desired
level of occlusion in the vasculature and the nature of the
aqueous solution. See the description of “Swelling
Behavior”.
• QuadraSphere Microspheres may be present outside the
vial. Therefore, the vial must be aseptically handled away
from the main sterile field.
• Ensure
the
compatibility
of
the
QuadraSphere
Microspheres with the intended size of catheter to be used.
See the table above.
• Inspect the packaging to confirm that it is intact. Remove
the vial from the pouch. The external surface of the vial is
sterile.
PREPARATION FOR EMBOLIZATION:
The approximate reconstitution time is 10 min.
• Fill a 10ml syringe with 100% NaCl 0.9% aqueous
solution or non-ionic contrast medium (or 50% NaCl 0.9%
aqueous solution and 50% contrast). Connect the syringe to
a needle of 20 gauge diameter or larger.
• To ensure proper reconstitution of the QuadraSphere
Microspheres, grasp the vial horizontally in your fingertips
and roll the vial several times. This will transfer the dry
contents of the vial to the sidewall.
Note: Pull back only the flip-top cap; do not remove the
crimp ring or the stopper from the vial.
• Carefully insert the needle from the syringe through the
stopper of the vial. Continue rolling the vial in your fingertips
and inject the full amount (10ml) of reconstitution medium
into the vial, then place the vial vertically and carefully
remove the syringe with the needle attached.
Note: The vial is hermetically closed. Proper aspiration
and/or venting techniques, as approved by the healthcare
facility, may be used for easier injection of reconstitution
medium into vial. If aspiration of air from the vial is
performed prior to reconstitution, exercise caution not to
remove the spheres from the vial.
• To ensure a homogeneous reconstitution of the
QuadraSphere Microspheres, gently invert the vial back and
forth so that the liquid contacts the stopper 5-10 times.
Dry (
µm)
Approximate
Reconstituted Size
range (
µm)
Catheter Size ID
(in.)
30-60
120-240
≥ 0.021
50-100
200-400
≥ 0.021
100-150
400-600
≥ 0.024
150-200
600-800
≥ 0.027
biosphere_notice Z 1822 REV J 06-12:1 23/07/12 16:53 Page 2
