Thermo Fisher Scientific Ion Selective Electrodes Sodium User Manual
Page 11
Instruction Manual
Sodium Electrode
9
Limits of Detection
The upper limit of detection in pure sodium solutions is 1M. In the presence of other ions, the upper
limit of detection is above 10
-1
M sodium, but two factors influence this upper limit. Both the
possibility of a liquid junction potential developing at the reference electrode and the salt extraction
effect influence this upper limit. Some salts may extract into the electrode membrane at high salt
concentrations, causing deviation from the theoretical response. Either dilute samples between 1M
and 10
-1
M or calibrate the electrode at 4 or 5 intermediate points.
Free sodium ion concentration down to 1.0X10-
6
M or 0.1 ppm can be measured in basic solutions.
For measurements below 10-
5
M or 1 ppm, use plastic lab-ware (and low level procedures) since a
significant pickup of sodium may occur from glassware due to removal from container walls.
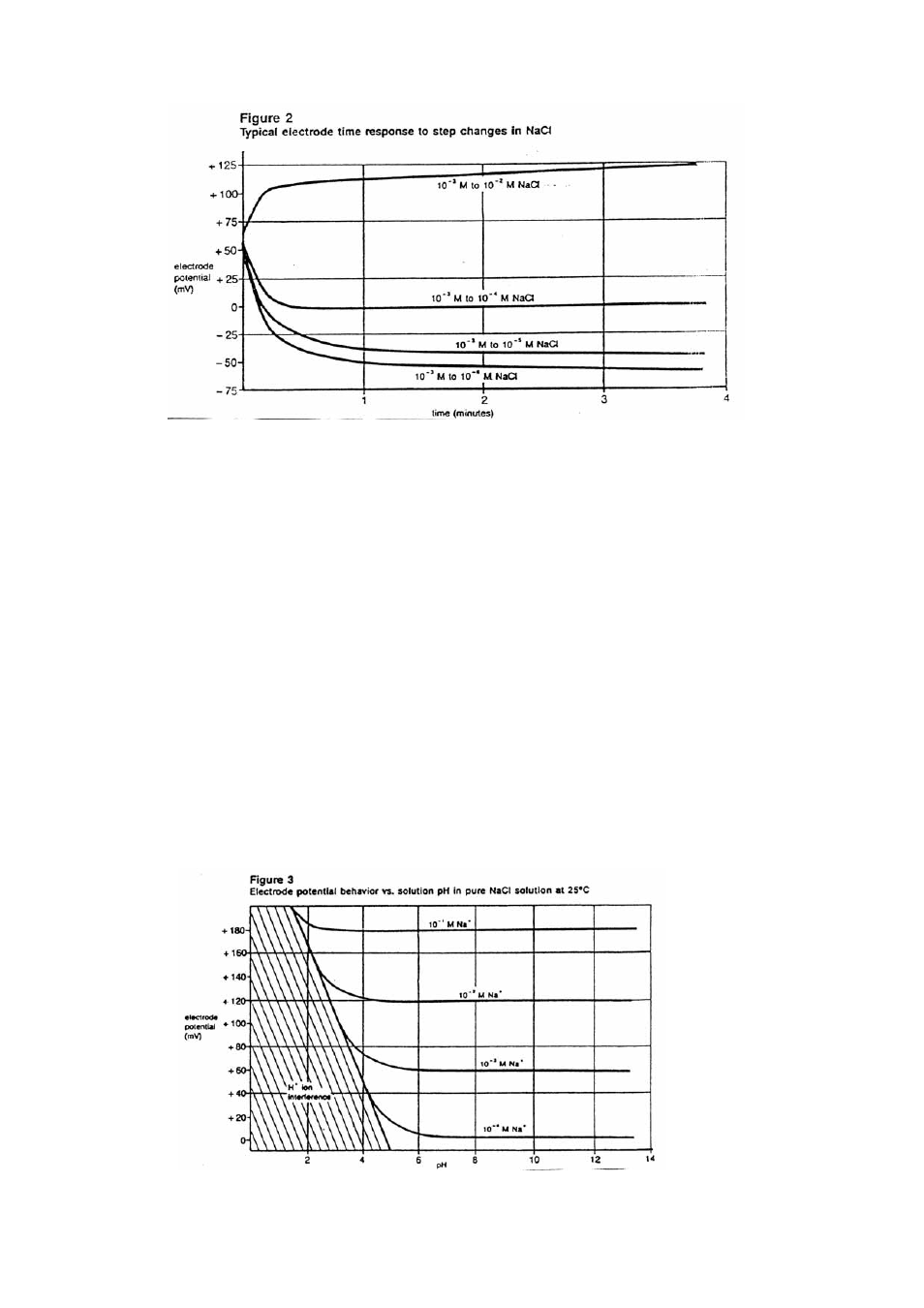
pH Effects
The electrode response to sodium ions is greatly influenced by the pH of the solution. Hydrogen
ion interferes with measurements of low level sodium ion measurements, although the electrode can
be used over a wide pH range. (See Figure 3.)
