Thermo Fisher Scientific Ion Selective Electrodes Ammonium User Manual
Page 10
Ammonium Electrode
Instruction Manual
4
TABLE
1: Concentration Unit Conversion Factors
ppm NH
4
+
ppm N moles/liter NH
4
+
1.80
1.40
1x10
-4
18.00
14.00
1x10
-3
180.00
140.00
1x10
-2
Measurement Procedure
Direct Measurement
A simple procedure for measuring a large number of samples. A single meter reading is all that is
required for each sample. The ionic strength of samples and standards should be made the same by
adjustment with ISA for all ammonium samples. The temperature of both sample solution and of
standard solutions should be the same.
Direct Measurement of Ammonium (using a standard pH/mV meter)
1. Prepare
10-
2
, 10-
3
, and 10-
4
M or 100, 10, and 1 ppm standards by serial dilution of the
0.1M or 1,000 ppm standard. Add 2 ml of ISA per 100 ml of standard.
2.
Place the most dilute solution (10-
4
M or 1 ppm) on the magnetic stirrer and begin stirring
at a constant rate. After assuring that the meter is in the mV mode, lower the electrode
tip(s) into the solution. When the reading has stabilized, record the mV reading.
3.
Place the midrange solution (10-
3
M or 10 ppm) on the magnetic stirrer and begin stirring.
After rinsing the electrode(s) with distilled water and blotting dry, immerse the electrode
tip(s) in the solution. When the reading has stabilized, record the mV reading.
4.
Place the most concentrated solution (10-
2
M or 100 ppm) on the magnetic stirrer and begin
stirring. After rinsing the electrode(s) with distilled water and blotting dry, immerse the
electrode tip(s) in the solution. When the reading has stabilized, record the mV reading.
5.
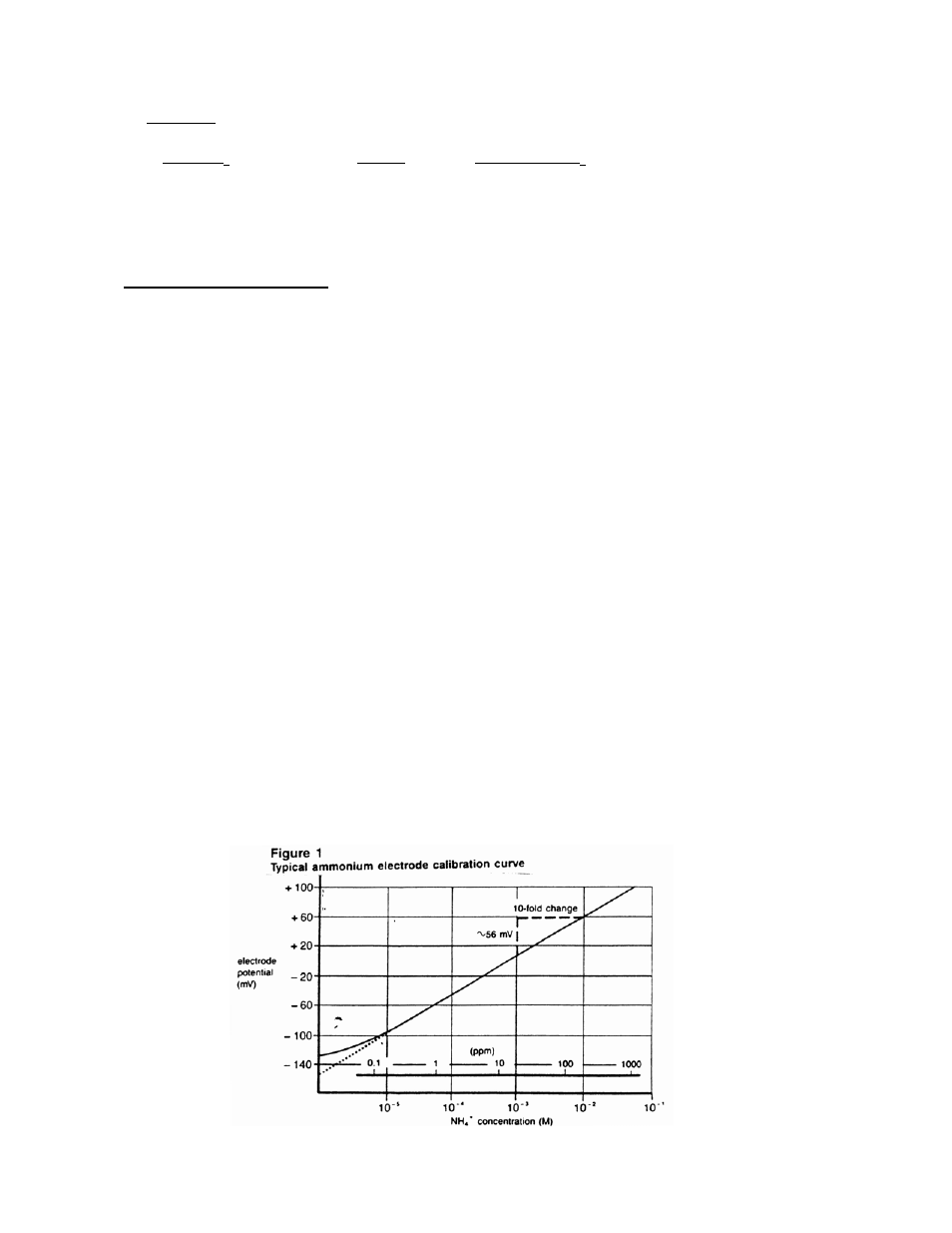
Using the semi-logarithmic graph paper, plot the mV reading (linear axis) against the
concentration (log axis). A typical calibration curve can be found in Figure 1.
