3B Scientific Electrochemistry Kit User Manual
Page 25
26
Experiment 10 - Measuring voltage Teacher's instructions
Assembly, charging and discharging of a steel accumulator
Chemicals Hazard
symbols
R phrases
S phrases
Equipment
Meter
Potassium hydroxide 20%
approx.
(
≈
4 molar)
22-35
26-36/37/39-45
Electrodes:
1 Ni, 1 Fe
Distilled water
---
---
2 Experiment cables
2
Pipettes
Warning: Be careful when handling potassium hydroxide! Wear protective glasses!
Experiment procedure:
1.
The suitably concentrated potassium hydroxide (20%) required for the experiment should be given to the students.
2.
Assemble the battery block as described.
3.
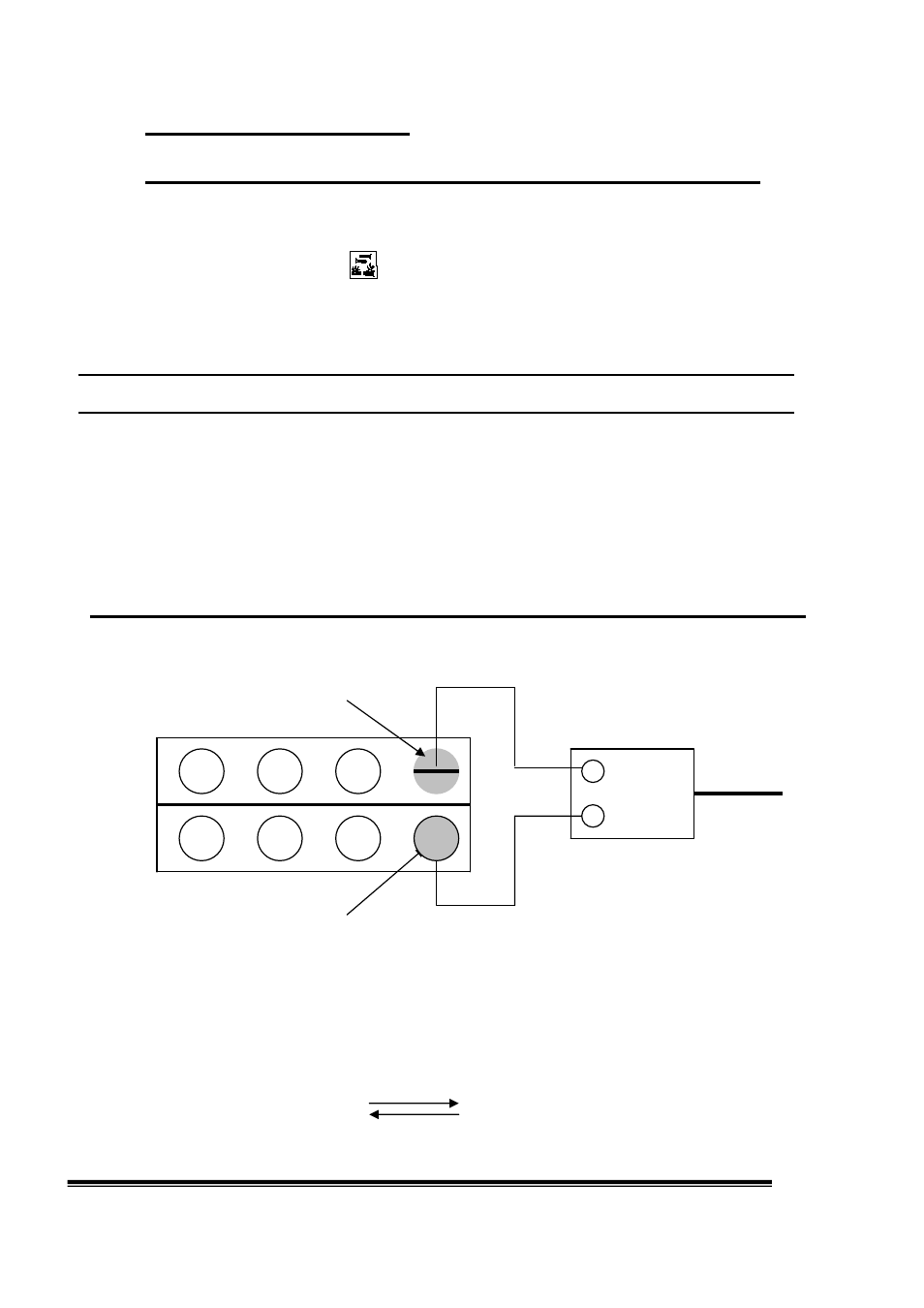
Fill two opposing half cell chambers with 20% potassium hydroxide.
4.
Insert an iron electrode into one chamber and a nickel electrode into the other.
5.
To charge up the cell, connect the 3V adapter so that the nickel electrode is connected to the + pole and the iron
electrode is connected to the - pole.
6.
After connecting the 3V adapter to the mains power supply and plugging the latter into the 230V mains, allow the
accumulator to charge up for about 10 minutes.
7.
The 3V adapter is then replaced by the meter and the voltage produced by the accumulator can then be measured.
Observation and evaluation:
Fe
20% potassium hydroxide
_ connection
3V adapter
+ 230V
20% potassium hydroxide Ni
The voltage displayed on the meter should be read off and written down. The no-load voltage at the terminals is
1.3 V approx.
If the meter is connected for a longer period of time, it can be observed that the voltage decreases rapidly because the capacitance of
this co-called Edison accumulator is very low.
The following chemical processes take place within the Edison accumulator:
Discharging
2 NiO(OH) + Fe + 2 H
2
O 2 Ni(OH)
2
+ Fe(OH)
2
Charging
