3B Scientific Electrochemistry Kit User Manual
Page 23
24
Experiment 9 - Measuring voltage Teacher's instructions
Measuring the voltage for various electrolyte temperatures
Chemicals Hazard
symbols
R phrases
S phrases
Equipment
Meter
Silver nitrate
34-50/53 26-45-60-61
Electrodes:
2 Ag
Distilled water
---
---
2 Experiment cables
1
Beaker
1 Bunsen burner
1
Thermometer
2
Pipettes
Warning: Be careful when handling silver nitrate! Silver nitrate is corrosive!
Experiment procedure:
1. The 0.01 molar silver nitrate solution should be given to the students.
2. Assemble the battery block as described.
3.
Put about 15 ml of 0.01 molar silver nitrate solution in a beaker and heat it to about 70-80°C .
4.
Fill one chamber of the battery block with 0.01 molar silver nitrate at room temperature and put the hot 0.01 molar
silver nitrate solution into the chamber opposite.
5.
Insert one silver electrode into both electrolyte solutions, connect the electrodes to the meter and read off the
voltage.
Observation and evaluation:
By contrast with the previous observation that no voltage can be measured between electrolytes of the same concentration, it can be
observed here that a voltage is indeed measured if the equally concentrated electrolytes are at different temperatures. This means that
differences in temperature between electrolyte solutions can have an effect on the resulting potential (theoretically about 2 mV/10K). In
the experiment a voltage of about 20 mV is measured. This gradually decreases as the temperatures of the two electrolyte solutions
converge. When the temperatures are equal, the measured voltage is 0 V.
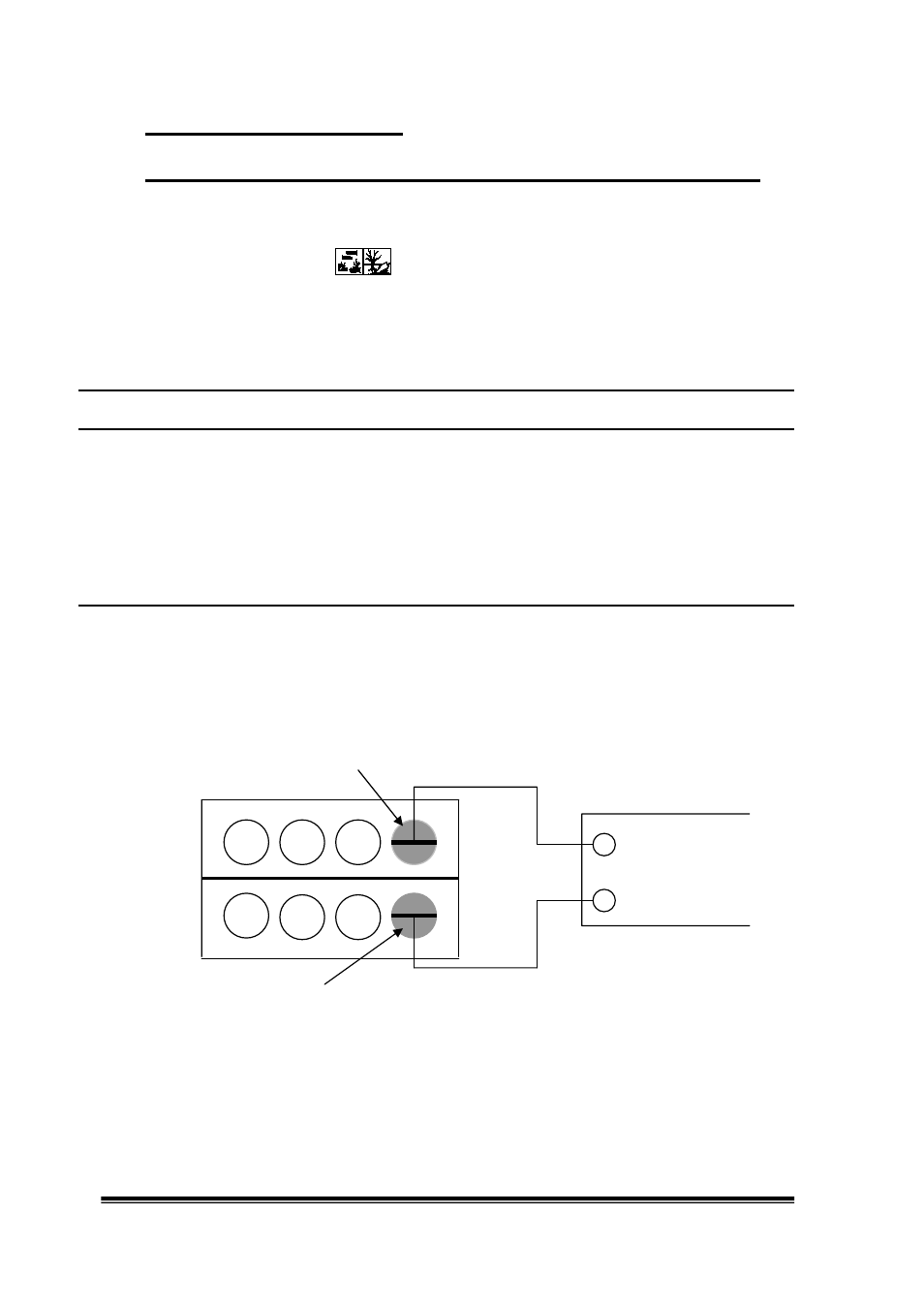
Silver nitrate solution at room temperature
+ +
0.01 mol/
l Meter
-
0.01 mol/l
Silver nitrate solution at 70-80°C
To make 1 liter of the electrolyte solution:
To make a 0.01 molar solution of AgNO
3
, 1.69 g of AgNO
3
should be dissolved in a liter of water.
