3B Scientific Air Cushion Plate User Manual
Page 24
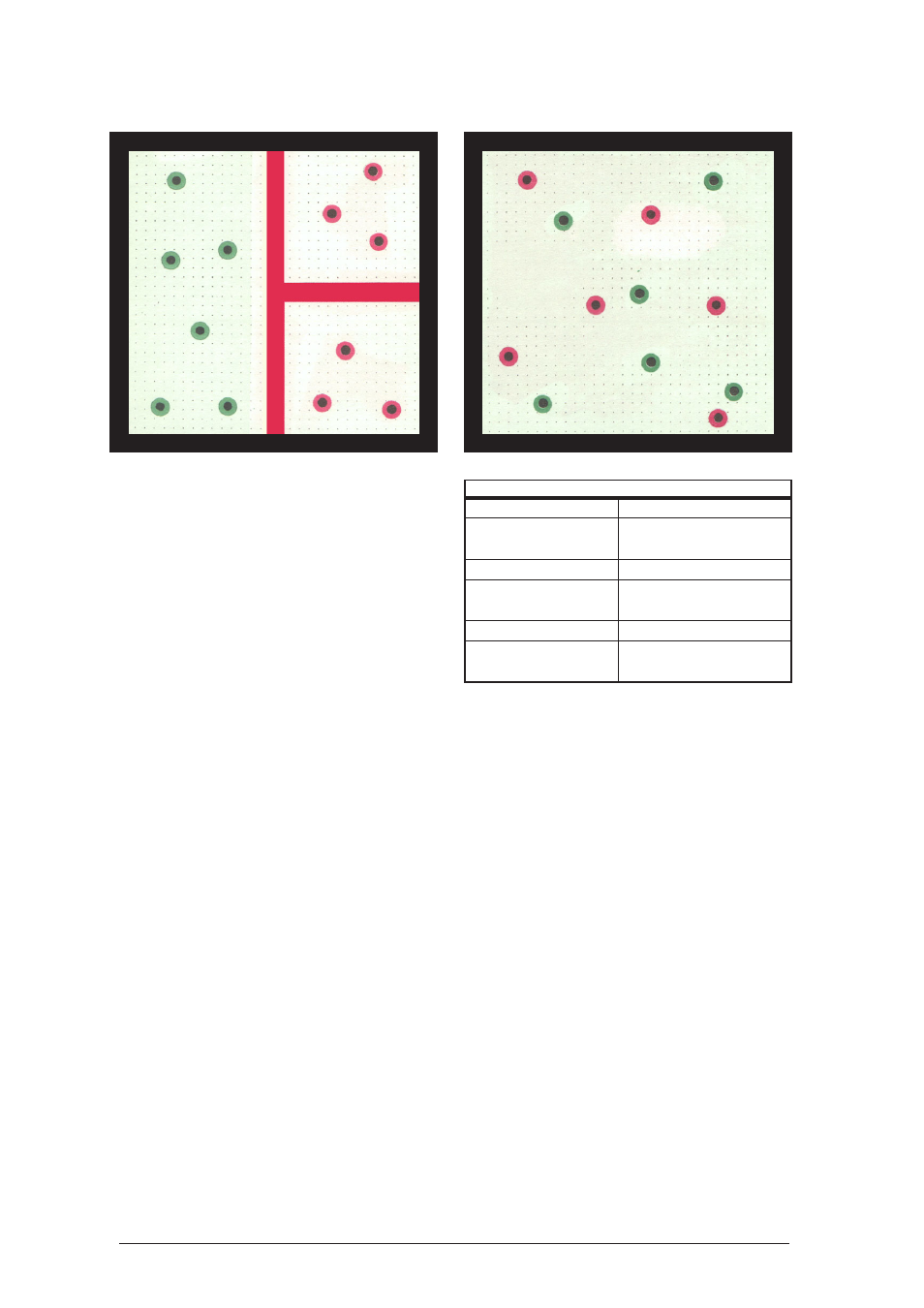
Physical Experiments on the Air-Cushion Table
24
Result:
After removing the partition, the hover discs mix
evenly as a result of their own motions. This will
occur more rapidly the higher the mean velocity
is.
Interpretation:
When removing the partition separating two gas-
es contained in a vessel, these gases will mix by
themselves (diffusion). This process is caused by
the thermal motion of the molecules. The higher
the temperature, the higher the diffusion speed.
The reason for this is the higher velocity of the
molecules at higher temperatures.
As a result of diffusion, the system changes from
a state of a higher order to that of a lower order.
The entropy increases.
2.1.15 Diffusion of a Gas through a Porous
Partition
Components:
Air-cushion table with fan
Overhead projector
Magnetic barrier, long
2 Pieces
Magnetic barrier, short
2 Pieces
Magnetic barrier with opening
l Piece
Hover disc, green
4 Pieces
Hover disc, red
6 Pieces
Model simulation
Real Object
Model
Vessel containing
Experiment surface
the gas
of the air-cushion table
Walls of the vessel
Magnetic barriers
Porous partition
Magnetic barrier with
opening
Molecules of one gas Red hover disc
Molecules of the
Green hover disc
other gas
How to proceed:
Align the air-cushion table horizontally and at-
tach the magnetic barriers. The magnetic barrier
with the opening divides the experiment surface
in two halves with its ends latching into the re-
cesses provided in barriers nos. 3 and 4. The red
discs are placed in the half that is delimited by
magnetic barrier no. 1 with the slit for the air-
flow entering from the side.
Select the lowest possible airflow at which the
discs are sure to float and observe their move-
ment through the opening of the middle barrier.
Repeat the experiment using a stronger airflow,
increasing the mean velocity of the hover discs
by repeatedly opening the impulse valve for a
short time, as opposed to the first experiment.
For the next experiment, place 4 green discs in
