Principle of strong acid water generation – Hoshizaki ROX-20TA-U User Manual
Page 8
6
U8AA5540012
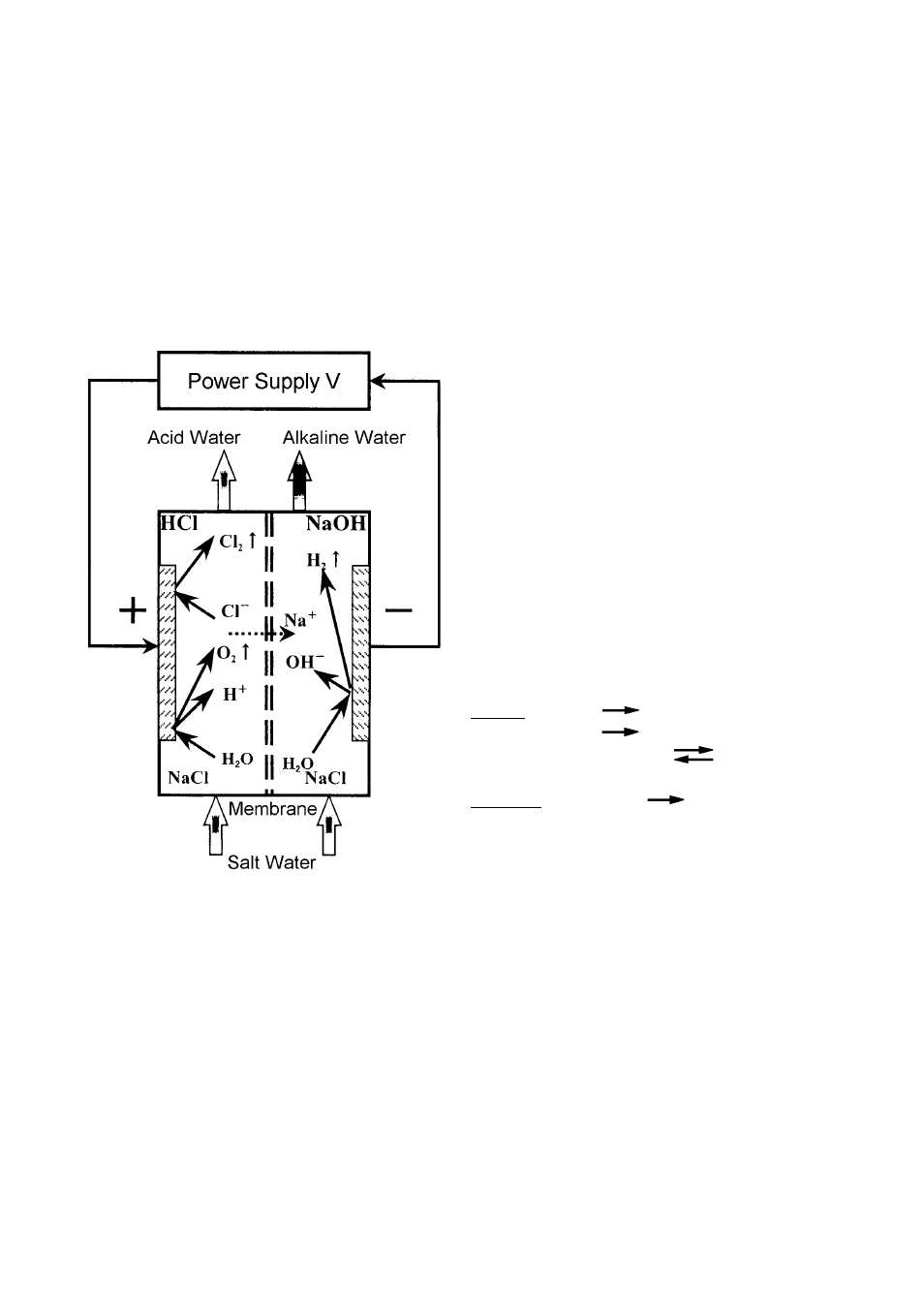
5. PRINCIPLE OF STRONG ACID WATER GENERATION
Strong acid water is generated at the anode inside the Electrolytic Cell where the membrane
divides the anode and cathode, by electrolyzation of 0.1% or less salt water. See the figure
below.
At the anode, chlorine ion (Cl
-
) generates chlorine gas which reacts with H
2
O to generate
chloride and hypochlorous acid (HOCl). H
2
O is also electrolyzed at the anode to become
oxygen (O
2
) and hydrogen ion (H+). Eventually, the anolyte pH falls below 2.7, the oxidation-
reduction potential (ORP) rises significantly, and the available chlorine concentration reaches
20 - 60 mg/L.
[Reaction Formula]
Anode
H
2
O
1/2O
2
+ H+ + 2e
-
2Cl
-
Cl
2
+ 2e
-
Cl
2(aq)
+
H
2
O
HCl + HOCl
Cathode
H
2
O + 2e
-
1/2H
2
+ OH
-
