Hanna Instruments HI 3896 User Manual
Page 6
6
For example, fungi prefer acidic conditions whereas most bacteria, especially those put-
ting nutrients at the plants’ disposition, have a preference for moderately acidic or slightly
alkaline soils. In fact, in strongly acidic conditions, nitrogen fixing and the mineralization
of vegetable residual is reduced.
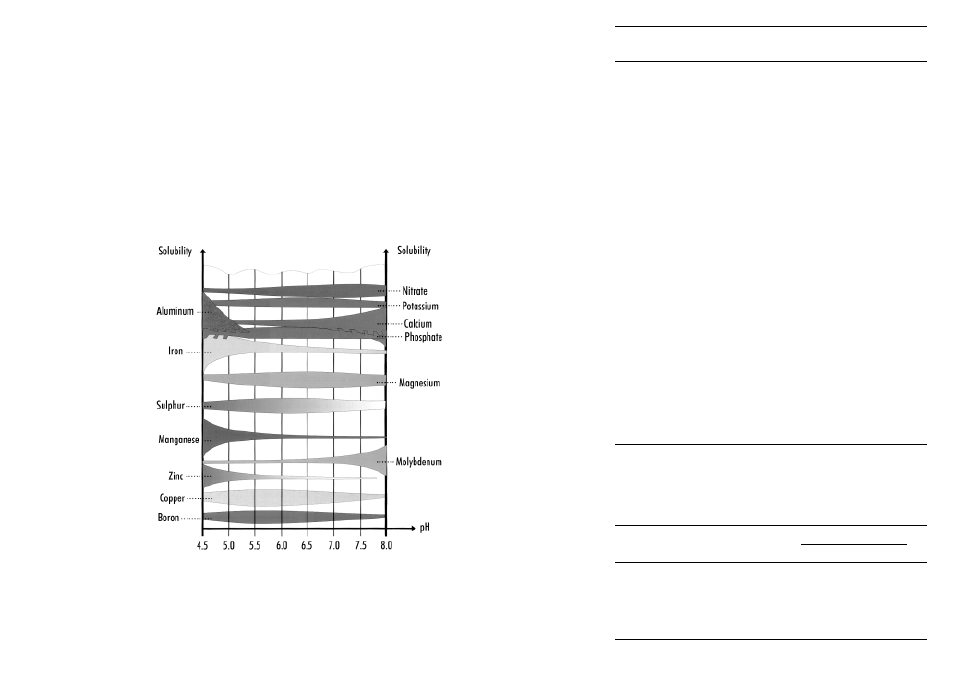
Plants absorb the nutrients dissolved in the soil water and the nutrient solubility depends
largely on the pH value. Hence, the availability of elements is different at different pH
levels (Fig. 4).
Each plant needs elements in different quantities and this is the reason why each plant
requires a particular range of pH to optimize its growth.
For example, iron, copper and manganese are not soluble in an alkaline environment. This
means that plants needing these elements should theoretically be in an acidic type of soil.
Nitrogen, phosphorus, potassium and sulfur, on the other hand, are readily available in a
pH range close to neutrality.
Fig. 4. Solubility of the
elements according to
varying pH values
Furthermore, abnormal pH values, increase the concentration of toxic elements for plants.
For example, in acid conditions, there can be an excess of aluminum ions in such quantities
that the plant can not tolerate. Negative effects on chemical and physical structure are
also present when pH values are too far from neutral conditions (break up of aggregates,
a less permeable and more compact soil).
11
Garlic
100
80
30
60
Lettuce
200
60
35
100
Maize (grain)
120
160
65
80
Melon
350
180
65
260
Onion
350
150
60
160
Pea
50
190
55
170
Pepper
250
100
35
130
Potato
350
140
55
220
Rice (whole plant)
60
100
45
95
Soybean
40
300
70
35
Spinach
250
120
40
130
Strawberry
150
165
60
265
Sunflower
30
130
45
145
Sugar beet
600
170
75
250
Tobacco (leaves)
24
85
55
230
Tomato
500
150
60
290
Watermelon
600
110
45
190
Soft Wheat (whole plant)
60
170
25
100
Hard Wheat (whole plant)
45
130
20
80
Apple
350
90
33
130
Apricot
150
110
35
125
Cherry
75
50
20
75
Grapevine
150
70
35
115
Grapefruit
300
130
45
180
Lemon
200
45
20
70
Olive
50
50
20
65
Orange
250
70
25
100
Peach
200
130
30
130
Pear
250
70
15
80
Plum
180
100
20
90
CROP
YIELD
Nitrogen
Phosphorus
Potassium
(q/ha)
N (kg/ha)
P
2
O
5
(kg/ha)
K
2
O (kg/ha)
Tab.6.
The relationship between dosages of fertilizer elements and their presence in the soil is
shown in Tab. 7. As above, the quantities reported are only indicative. Chemical
analysis can be used as a basis for the evaluation, however other factors connected with
the production also need to be considered.
CROP
SOIL CONTENT
ADVISED DOSES (kg/ha)
N
P
2
O
5
K
2
O
Alfalfa
very low
0
150
230
low
0
130
150
medium
0
100
120
medium-high
0
80
90
high
0
60
60
very high
0
40
40
Tab. 7. Relation between
dosages of fertilizer
elements and their presence
in the soil
