Principle of operation – Hanna Instruments HI 95711 User Manual
Page 6
6
Absorption of Light is a typical phenomenon of interaction between electromagnetic radiation and
matter. When a light beam crosses a substance, some of the radiation may be absorbed by atoms,
molecules or crystal lattices.
If pure absorption occurs, the fraction of light absorbed depends both on the optical path length
through the matter and on the physical-chemical characteristics of the substance according to the
Lambert-Beer Law:
-log I/I
o
=
ε
λ
c d
or
A =
ε
λ
c d
Where:
-log I/I
o
= Absorbance (A)
I
o
= intensity of incident light beam
I
= intensity of light beam after absorption
ε
λ
= molar extinction coefficient at wavelength
λ
c
= molar concentration of the substance
d
= optical path through the substance
Therefore, the concentration "c" can be calculated from the absorbance of the substance as the
other factors are known.
Photometric chemical analysis is based on the possibility to develop an absorbing compound from
a specific chemical reaction between sample and reagents. Given that the absorption of a
compound strictly depends on the wavelength of the incident light beam, a narrow spectral
bandwidth should be selected as well as a proper central wavelength to optimize measurements.
The optical system of Hanna's HI 95 series colorimeters is based on special subminiature tungsten
lamps and narrow-band interference filters to guarantee both high performance and reliable
results.
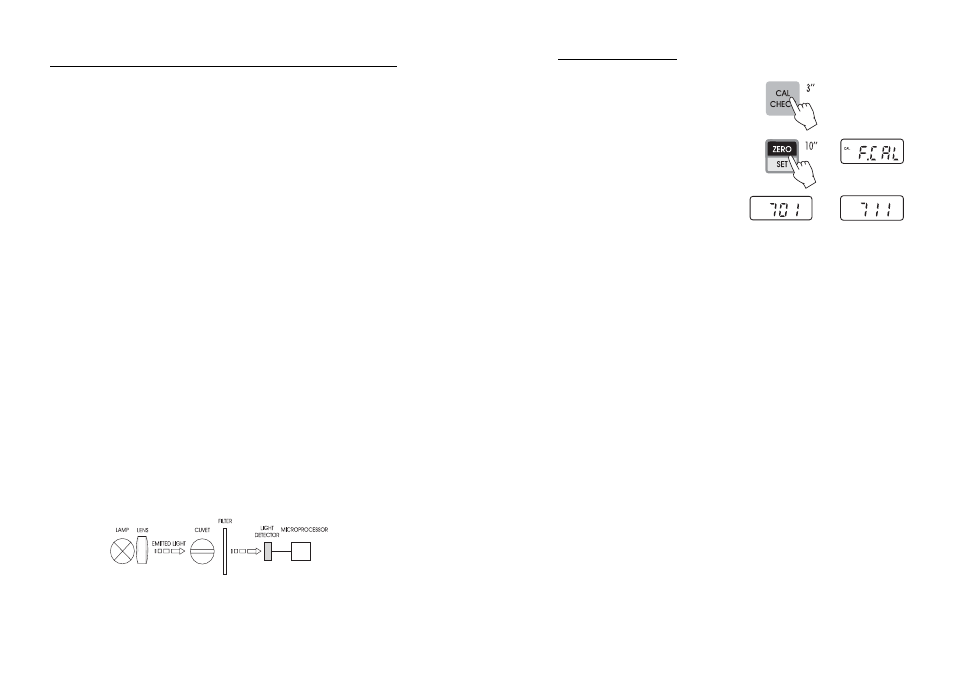
Block diagram (optical layout)
PRINCIPLE OF OPERATION
19
or
FACTORY CALIBRATION RESET
It is possible to restore factory calibration:
• Enter the calibration mode by holding CAL CHECK
for three seconds.
• Hold ZERO/SET for 10 seconds. The display
will show for 2 seconds “F.CAL” and the
parameter code ("701" or “711”) appears.
The factory calibration is automatically restored
and the instrument is ready for measurement.
