Bio-Rad Human Metabolic and Hormone Assays User Manual
Page 2
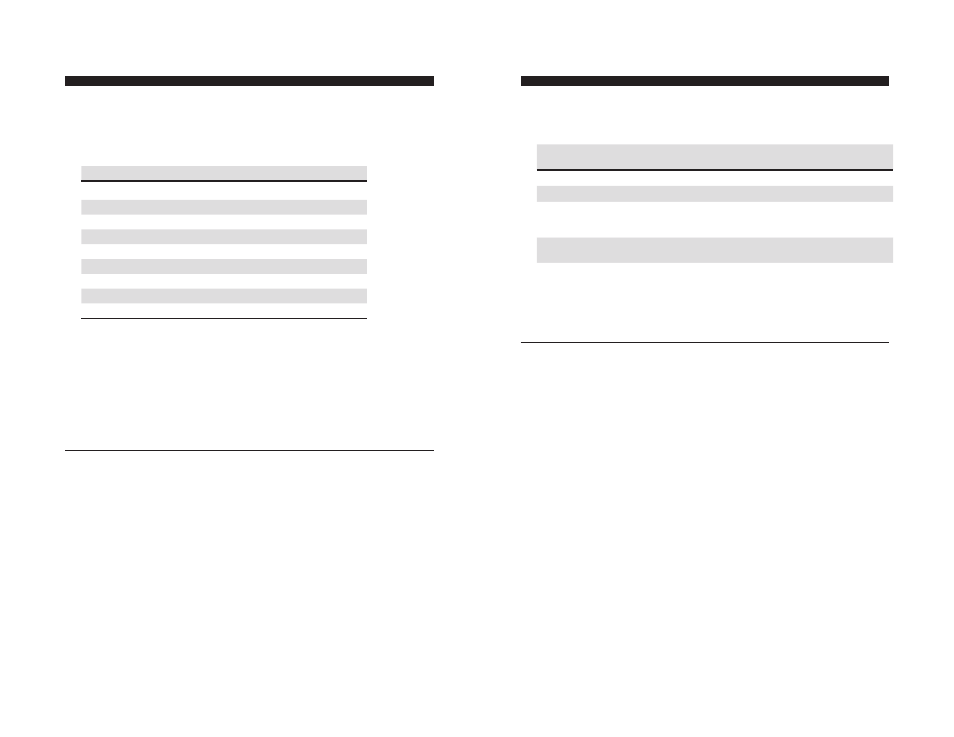
3. Add the appropriate amount of the standard diluent into the labeled
tubes according to the table below (this will be sufficient for duplicate
standard curves and blanks).
Standard
Volume of Standard Diluent, µl
Volume of Standard, µl
S1
––
150 from reconstituted vial
S2
100
50 of S1
S3
100
50 of S2
S4
100
50 of S3
S5
100
50 of S4
S6
100
50 of S5
S7
100
50 of S6
S8
100
50 of S7
Blank
100
––
4. Prepare working standards (S2–S8) by serial dilution. Transfer the
appropriate volume of standard into each of the labeled tubes with
standard diluent, as outlined above.
5. Vortex each standard at a medium setting before proceeding with the next
serial dilution. Change pipet tip at each dilution step.
6. The Blank tube consists of standard diluent alone.
C. Sample Preparation
1. Centrifuge serum or plasma samples at 1,000 x g for 15 min at 4°C to
remove particulates from all samples prior to use.
2. Prepare sample dilutions in 0.5 ml or 1.0 ml polypropylene tubes, as
required for the assay.
Bio-Plex Pro RBM Metabolic and Hormone Assays Quick Guide
Bio-Plex Pro RBM Metabolic and Hormone Assays Quick Guide
3. Dilution scenarios provided below are sufficient to run each sample in
duplicate.
Panel
Sample
Dilution
Volume of Sample, µl
Volume of Sample
Buffer, µl
Metabolic panel 1
1:5
20
80
Metabolic panel 2
1:5
20
80
Metabolic panel 3
1:500,000
(a) Prepare 1:50 5
(b) Prepare 1:100 5 of (a)
Prepare 1:100 5 of (b)
245
495
495
Metabolic panel 4
1:500
(c) Prepare 1:10 10
Prepare 1:50 10 of (c)
90
490
Hormone panel 1
1:5
20
80
Note: Controls are ready to use after reconstitution. No further dilution
is needed.
D. Dispensing of Reagents
1. Add 10 µl of blocker to all wells of the plate.
2. Add 30 µl of the standard, control, sample, or blank to the appropriate
well of the plate.
3. Vortex the capture beads at medium speed for 10–20 sec. Add 10 µl of
the beads to all wells of the plate.
4. Cover plate with plate seal and protect from light with aluminum foil.
Incubate on shaker at 850 ± 50 rpm for 1 hr at RT.
5. Wash the plate three times with 100 µl 1x assay buffer.
6. Vortex the reconstituted detection antibodies at medium speed for
10–20 sec. Add 40 µl to each well.
7. Cover and incubate at 850 ± 50 rpm, as in step 4, for 1 hr at RT. Do not
aspirate after incubation.
8. Prepare the required dilution of streptavidin-PE (SA-PE), as outlined in the
following table.
Note: Volumes in the table are for an entire 96-well plate. Smaller volumes
can be prepared, provided that the dilution ratios are maintained.
