5 influence of dna/rna, 3 optimizing the parameters – Eppendorf Multiporator - Electroporation User Manual
Page 11
35
For most cell types, the 20- to 30-minute incubation period in hypoosmolar buffer, which is unavoidable due to the
conditions of the experiment, has no effect on the viability of the cells. However, the incubation in hypoosmolar buffer
may induce apoptosis, or even lysis, in very sensitive cells. Therefore, it is strongly recommended to test the tolerance of
the cells to hypoosmolar conditions. The easiest way of doing so is by incubating the cells for 30 minutes in hypoosmolar
buffer and then performing a viability stain using trypan blue or propidium iodide. If observation under a microscope
reveals lysis in more than 10 % of the cells, the osmolarity of the buffer must be increased by adding isoosmolar buffer.
To determine the optimal osmolarity, it is advisable to incubate the cells in different mixing ratios of hypo- and isoosmolar
buffer for 30 minutes prior to the experiment (see Table 1, page 13). This 30-minute period is the maximum incubation
time for the cells in the electroporation buffer system. A new viability test followed by observation under a microscope
determines the osmolarity that can be tolerated by the cells. The mixing concentrations can then be used for all
subsequent experiments with this cell type.
Irrespective of the buffer system selected, it is essential to ensure that the cells do not remain in the electroporation
buffer for longer than 30 minutes.
The transfection efficiency of electroporation can be affected by the concentration, the purity and the size of the
molecules used.
a)
Influence of nucleic acid concentration
With the optimal electroporation parameters (osmolarity, voltage, pulse length), the quality of the results obtained at
plasmid concentrations between 5 µg/ml and 20 µg/ml is usually satisfactory.
The efficiency of the transfection may be raised by increasing the nucleic acid concentration, but only within a limited
concentration range. Tests with various different cell lines have shown that only in very few cases (e.g. when large
plasmids were used) plasmid concentrations in excess of 20 µg/ml lead to an increase in the transfection rate
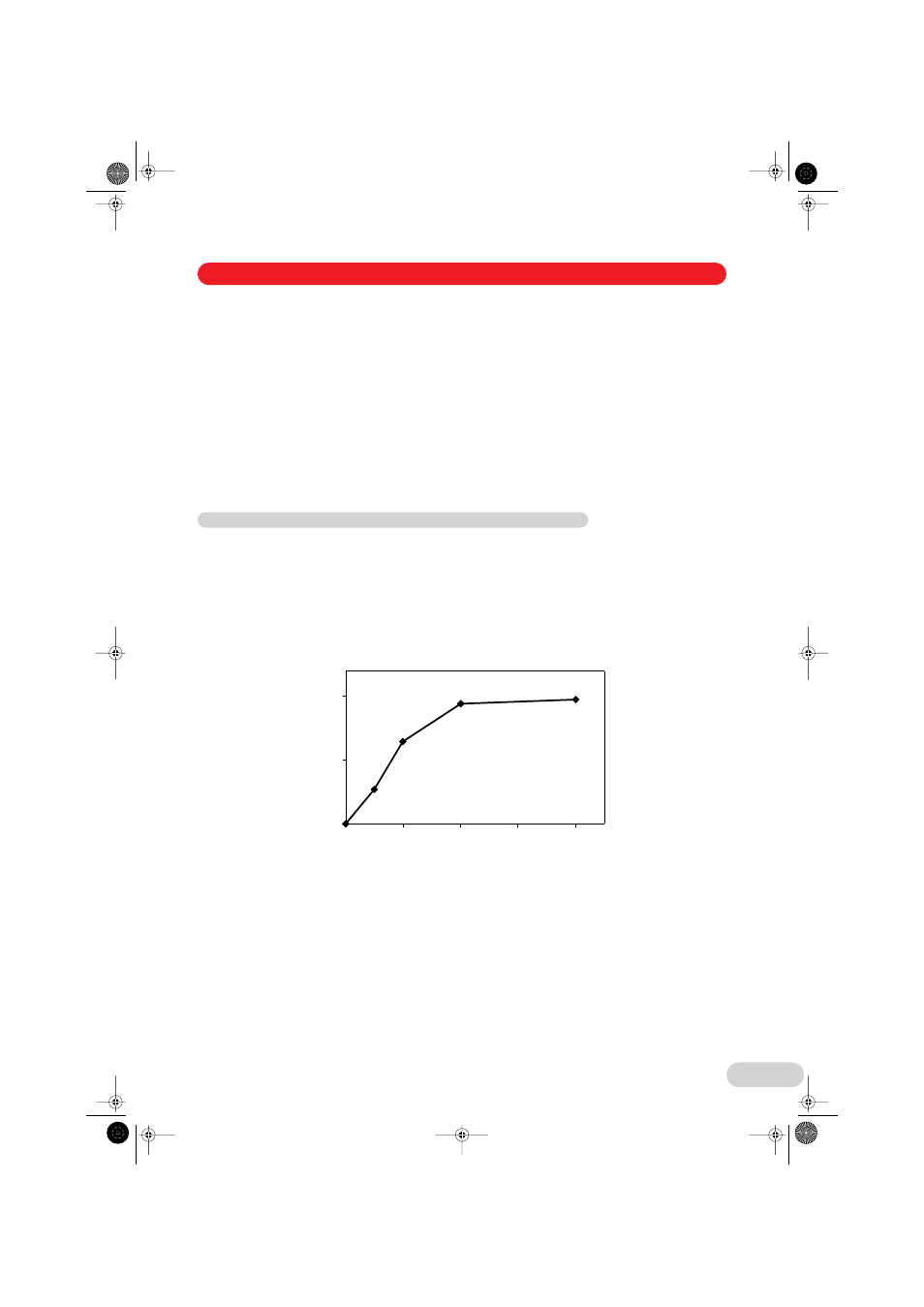
(see Fig. 3).
Fig. 3: Transient transfection efficiency in NIH-3T3 cells in relation to the DNA concentration (µg/ml).
The cells were electroporated with different concentrations of the pEGFP-N1 plasmid.
The transfection rate (max. = 100 %) was determined by FACS analysis.
3.5 Influence of DNA/RNA
Plasmid DNA [µg/ml]
T
ransient EGFP-N1 expr
ession [%]
0
50
100
0
10
20
30
40
3 Optimizing the parameters
3 Optimizing the parameters
Multipor_Appli_E_poration_en.fm Seite 35 Montag, 30. Januar 2006 2:17 14
