Thermo Fisher Scientific Ion Selective Electrodes Potassium User Manual
Page 11
Instruction Manual
Potassium Electrode
11
TABLE
4: Temperature vs Value for the Electrode Slope
Temp (
o
C) "S"(slope)
0
54.20
10
56.18
20
58.16
25
59.16
30
60.15
40
62.13
50
64.11
Electrode Response
Plotting the electrode mV potential against the potassium concentration on semi-logarithmic paper
results in a straight line with a slope of about 56 mV per decade. Refer to Figure 1.
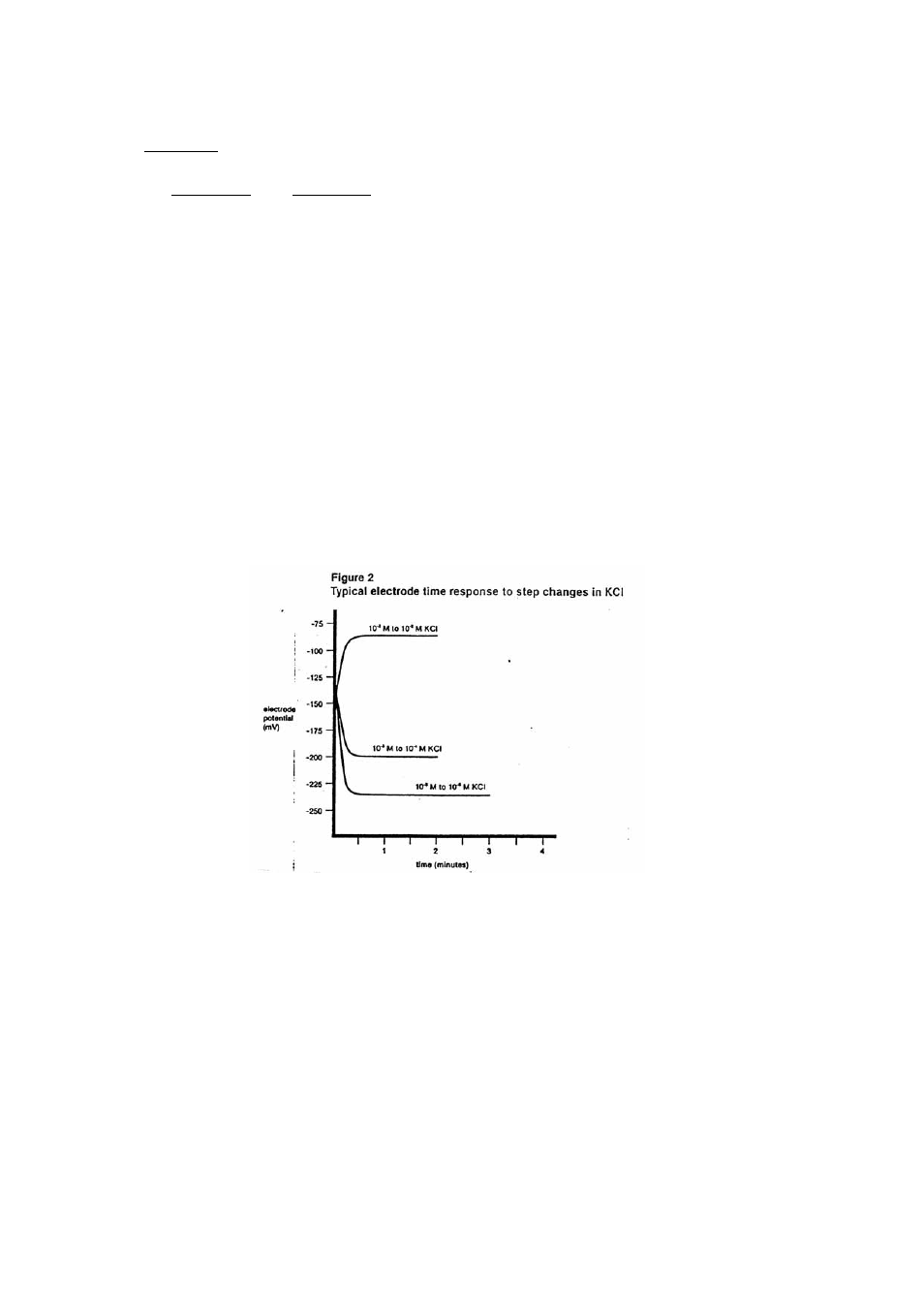
The time needed to reach 99% of the stable electrode potential reading, the electrode response time,
varies from one minute or less for potassium concentration above 1.0X10
-5
M to several minutes
near the detection limit. Refer to Figure 2.
Detection Limit
The upper limit of detection is 1M in pure potassium chloride solutions. The upper limit of
detection is above 1.0X10
-1
M when other ions are present, but the possibility of a liquid junction
potential developing at the reference electrode and the "salt extraction effect" are two limiting
factors. Some salts may be extracted into the electrode membrane at high salt concentrations
causing deviation from theoretical response. Calibrate the electrode at four or five intermediate
points, or dilute the sample, to measure samples between 1.0X10
-1
M and 1M.
The slight water solubility of the ion exchanger in the sensing module, which causes deviation from
theoretical response, determines the lower limit of detection. The theoretical response at low levels
of potassium chloride compared to actual response is shown in Figure 1. A low level measurement
is recommended if potassium measurements are made below 1.0X10
-5
M (0.39 ppm as potassium).
