Experiment 3: ethanol fuel consumption, Experiment 2: exploring polarity – Velleman KNS13 User Manual
Page 3
Experiment 4: Exploring the effect of varying fuel concentrations
You can make the different concentrations of ethanol fuel in the initial mix. For a 15% solution, add 9 ml of pure ethanol and fill water
to the level of 60 ml. You can use a multi-meter or Horizon’s fuel cell software adaptor product ref. FCJJ-24 to measure the voltage
difference produced by the fuel cell. Through experimentation, you will find that increasing or decreasing the concentration of the Ethanol
does not noticeably make the fan run faster.
The reason for this is that the capability of the catalyst used on proton exchange membrane in the fuel cell is limited. Similarly to many
people going through a narrow door, the speed of people going through the door is determined by the width of the door, but not by the
amount of people.
Warning: The safe experimentation range for the Bio-Energy Kit is within ethanol concentrations ranging from 5-15%. Please
note that the concentration cannot be higher than 15-20% otherwise it will permanently damage the fuel cell.
Tip: If the device will not be used for more than one day, first pour out the solution in the container and then purge out all the remaining
solution in the fuel cell by pouring purified or distilled water in the container. Make sure the purging valve is switched to the right side.
Make sure all of the purified or distilled water flows out of the container. Do not let the solution stay in the fuel cell otherwise it will
damage the fuel cell.
Experiment 3: Ethanol fuel consumption
When the fan begins to run slower or stops running completely, this means
the ethanol present in the fuel cell chamber is mostly consumed. In normal
temperature conditions, the majority of the ethanol inside the fuel cell
chamber turns into acetic acid, which is the main component of vinegar.
Let’s investigate the consumed fuel (acetic acid) when the fan begins to run
slowly.
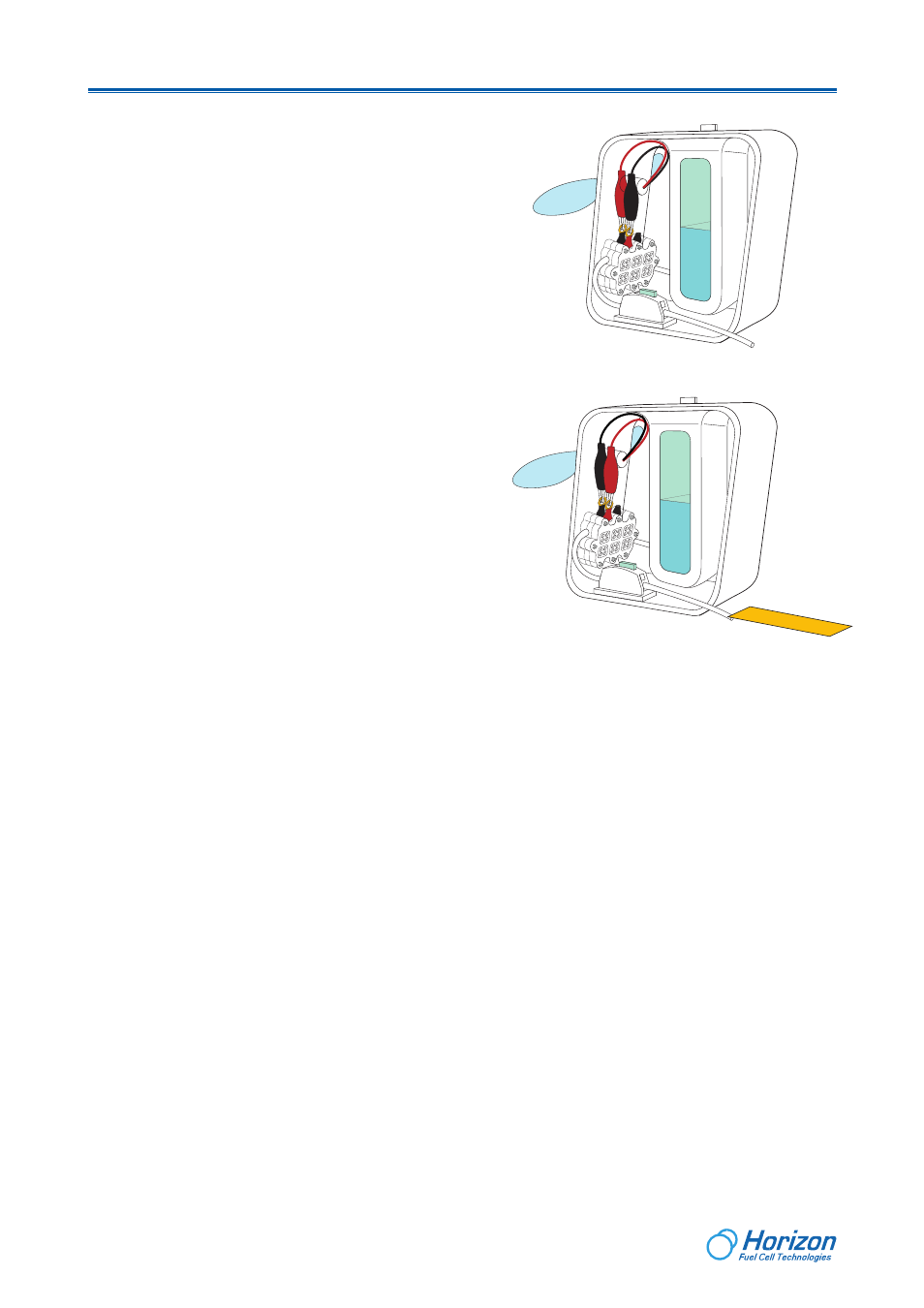
Step 1: Place a piece of PH paper under the outlet of the purging tube.
Step 2: Open the valve slowly by sliding the switch towards right side, and
release a drop of the solution onto the pH paper, and then close the valve.
You can see the paper color changing to a reddish color quickly.
Step 3: Dip a new pH paper into the solution container. You will notice that
the color of the PH paper changes very little.
The difference in pH paper coloring indicates the change of the acidity level. Ethanol turns into acetic acid during the reaction taking
place at anode side of the fuel cell, and the pH of the solution noticeably changes from pH level 6 to pH level 2 showing a red color. The
chemical reactions taking place at the anode and summarized on page 8 show that acetic acid is formed as hydrogen protons depart
from the ethanol molecule and the water molecule. These hydrogen protons cross the fuel cell membrane, and the liberated electrons
form the electricity that is able to propel the fan.
Conclusion: The Direct Ethanol Fuel Cell creates electricity by chemically converting the ethanol solution into an acid solution, which is
close to common vinegar. In order for the fuel cell to function continuously, “spent” fuel must be replaced with new fuel regularly.
Experiment 2: Exploring polarity
Step 1: Connect the positive (red) crocodile clip to the positive side of the
fuel cell (red “+” mark), then connect the negative (black) crocodile clip to
the negative side of the fuel cell (black “-” mark).
You will notice the fan will turn clockwise.
Step 2: Now repeat the process, this time however connect the positive
(red) crocodile clip to the negative side of the fuel cell (black “-” mark) and
connect the negative (black) crocodile clip to the positive side of the fuel cell
(red “+” mark). You will notice the fan will turn counter-clockwise.
Conclusion: The current flows from positive to negative, creating a
clockwise spin of the fan. By inverting the polarity connections, the current
flow reverses and makes the fan spin in the opposite direction.
