Features, System requirements, Indications for use – GE Healthcare Innova Vision User Manual
Page 2: Regulatory compliance
© 2012 General Electric Company.
All rights reserved. Data subject to change.
GE and GE Monogram are trademarks of General Electric Company.
* Trademark of General Electric Company.
Features
•
Overlay 3D models from CT, MR or 3D
rotational images on live fluoroscopic
image.
•
3D models acquired in GE
interventional suite are automatically
registered when exported on live
fluoroscopic images.
•
Directly register CT and MR 3D models
with only 2 fluoroscopy views for
efficiency in procedure time and dose
•
Registration is adjusted in real time
along the procedure, following the
movement of frontal gantry, source-to-
image distance, field of view, image
flip, and table motion.
•
Correct patient motion at table side or
in control room.
System Requirements
•
AW VolumeShare 4
•
In-room AW monitor
Indications for Use
Innova Vision is intended to enable
users to load 3D datasets and
overlay and register in real time
these 3D datasets with fluoroscopic
or radiographic images of the same
anatomy in order to support
catheter/device guidance during
interventional procedures.
Regulatory Compliance
This product complies with the
European Council Directive
93/42/EEC Medical Device Directive
as amended by European Council
Directive 2007/47/EC.
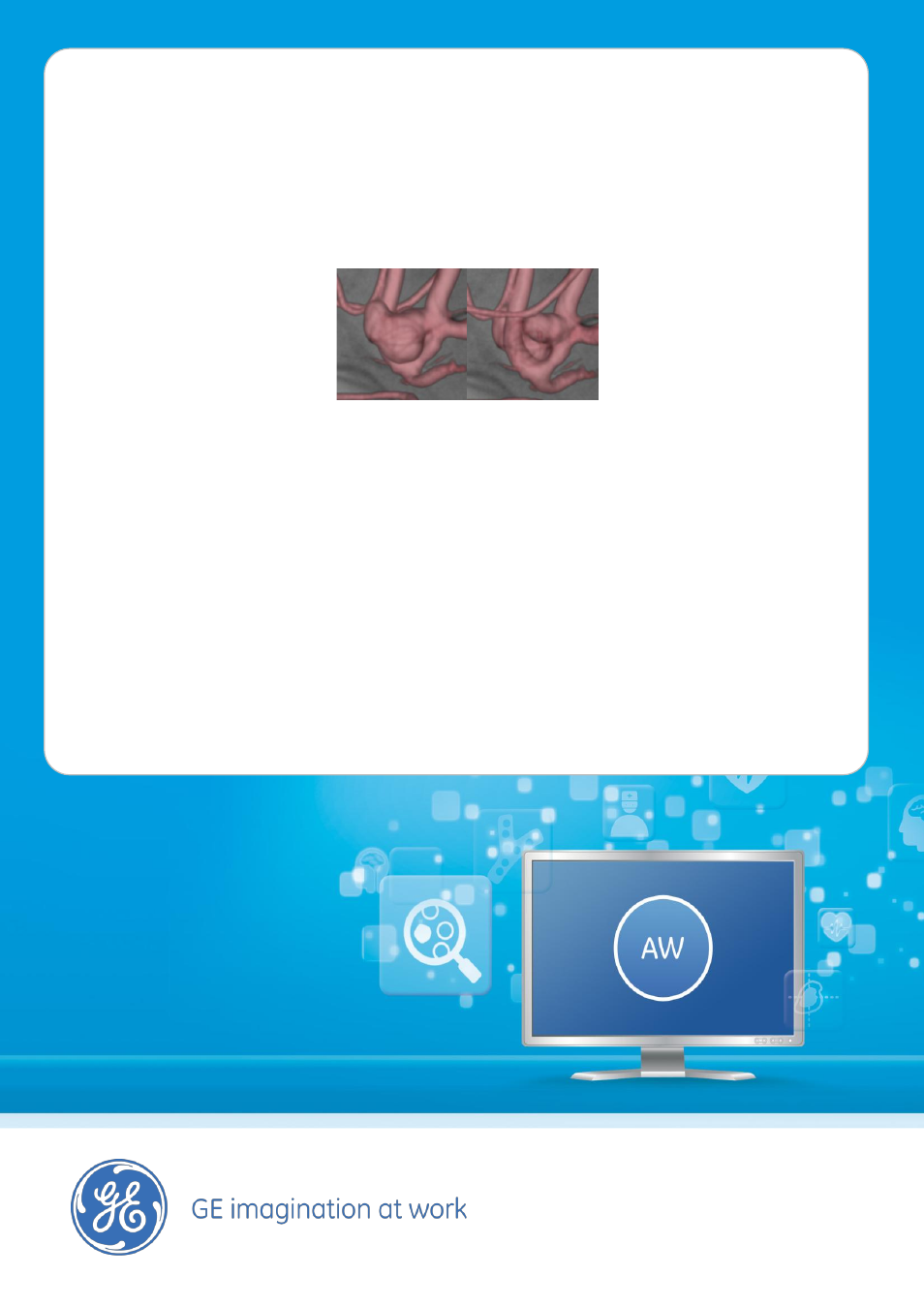
•
A variety of tools enhance
visualization and guidance capability:
- With Backview visualize 3D model
from the opposite side without
losing registration on the
fluoroscopy and moving the
gantry:
- Adjust 3D model opacity and
brightness to fit your preferences.
- Display user-defined landmarks.
- Digital Zoom
Fontal view
Backview
