Hanna Instruments HI 38079 User Manual
Page 2
The chemicals contained in this kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheet before performing this test.
HEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETY
REFERENCES
REFERENCES
REFERENCES
REFERENCES
REFERENCES
Adaptation of Standard Methods for the Examination of
Water and Wastewater
, 18
th
edition, 1992, APA AWWA WEF.
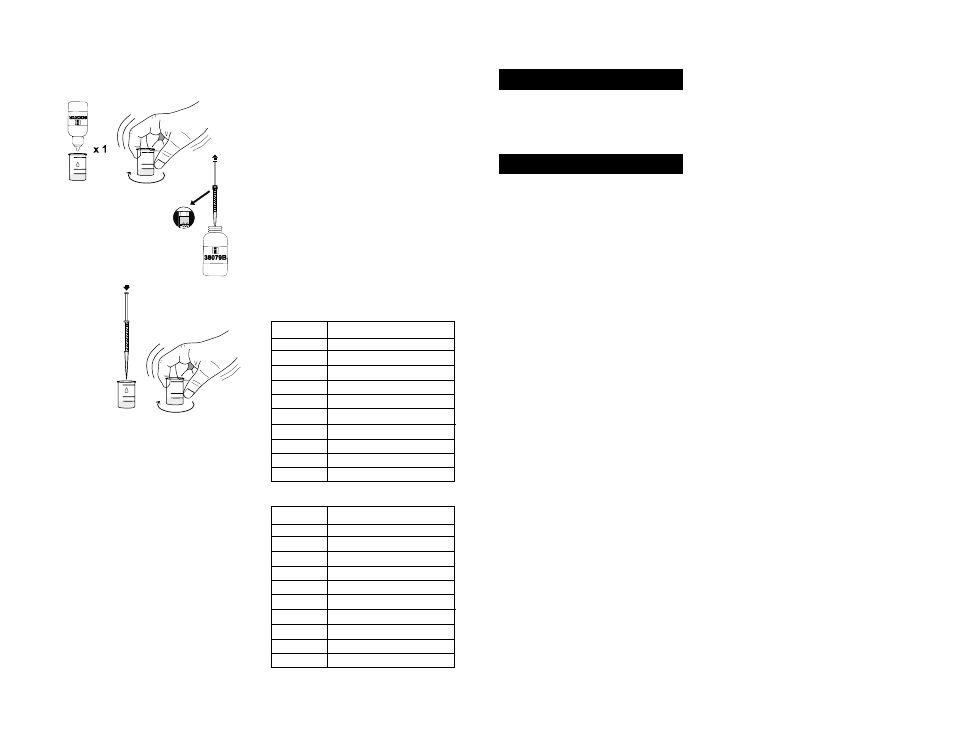
14- Add 1 drop of Calmagite Indicator and swirl to mix; if
magnesium is present, the solution will turn wine red.
15- Take the syringe and push the
plunger completely into the syringe.
Insert tip into the HI 38079B-0
EDTA Solution bottle and pull the
plunger out until the lower edge of
the seal is on the 0.0 mL mark of
the syringe.
16- Slowly add the
titration solution
drop by drop,
swirling after
each drop.
17- As the color changes from pink to purple, swirl for 15
seconds after each additional drop, until the solution
turns pure blue. Read off the milliliters of titration
solution from the syringe.
18- Calculate the mg/L (ppm) of Magnesium in your sample
as follows:
ppm of Mg = mL of titrant x 243
19- If your sample requires more than 1 mL of titrant to
turn pure blue, repeat the test from step 11 using, in
this case, 1 mL of filtered water sample, instead of 3
mL.
20- Follow then the instructions from step 12 to 17.
21- Calculate the mg/L (ppm) of Magnesium in your sample
as follows:
ppm of Mg = mL of titrant x 729
22- To convert the reading in mg/L of CaCO
3
, multiply the
ppm of magnesium by 4.114.
23- Rinse all labware with demineralized water after each
analysis and shake dry.
Note: High amounts of copper in your sample will alter the
final endpoint color. The solution will change from wine
red to purple without turning pure blue. In this case
add drops of titrant until no visible change in color is
obtained.
CONVERSION TABLES
for 3 mL sample:
mL of titrant ppm as Mg
ppm as CaCO
3
0.1
24
100
0.2
49
200
0.3
73
300
0.4
97
400
0.5
122
500
0.6
146
600
0.7
170
700
0.8
194
800
0.9
219
900
1.0
243
1000
for 1 mL sample:
mL of titrant ppm as Mg
ppm as CaCO
3
0.1
73
300
0.2
146
600
0.3
219
900
0.4
292
1200
0.5
365
1500
0.6
437
1800
0.7
510
2100
0.8
583
2400
0.9
656
2700
1.0
729
3000
