Hanna Instruments HI 83205-2008 User Manual
Page 16
30
31
Chlorine Dioxide
CHLORINE DIOXIDE
SPECIFICATIONS
Range
0.00 to 2.00 mg/L
Resolution
0.01 mg/L
Accuracy
±0.10 mg/L ±5% of reading
Typical EMC
±0.01 mg/L
Deviation
Light Source
Tungsten lamp with narrow band interference filter @ 575 nm
Method
Adaptation of the Chlorophenol Red method. The reaction between chlorine dioxide and
reagents causes a colorless to purple tint in the sample.
REQUIRED REAGENT
Code
Description
Quantity
HI 93738A-0
Reagent A
1 mL
HI 93738B-0
Dechlorinating Reagent B 1 packet
HI 93738C-0
Reagent C
1 mL
HI 93738D-0
Reagent D
1 mL
REAGENT SETS
HI 93738-01 Reagents for 100 tests
HI 93738-03 Reagents for 300 tests
For other accessories see page 73.
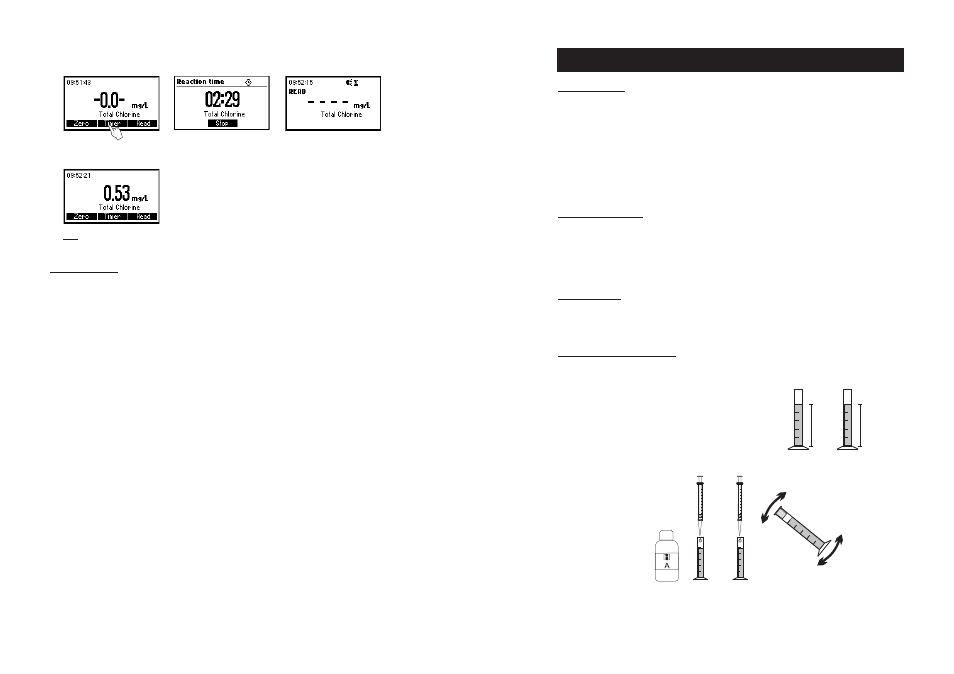
MEASUREMENT PROCEDURE
• Select the
Chlorine Dioxide method using the procedure described
in the
Method Selection section (see page 12).
• Fill two graduated mixing cylinders (#1 & #2) up to the
25 mL mark with the sample.
• Add 0.5 mL of HI 93738A-0 Chlorine Dioxide Reagent to each
cylinder (#1 & #2), close them and invert several times to mix.
#2
25 ml
#1
25 ml
#1 & #2
#1
#2
• Press TIMER and the display will show the countdown prior to the measurement or, alternatively, wait
for 2 minutes and 30 seconds and press READ. When the timer ends the meter will perform the reading.
• The instrument displays the results in mg/L of total chlorine.
Note: free and total chlorine have to be measured separately with fresh unreacted samples following the
related procedure if both values are requested.
INTERFERENCES
Interference may be caused by: Bromine, Iodine, Ozone, Oxidized forms of Chromium and Manganese.
In case of water with hardness greater than 500 mg/L CaCO
3
, shake the sample for approximately 2
minutes after adding the powder reagent.
In case of water with alkalinity greater than 250 mg/L CaCO
3
or acidity greater than 150 mg/L CaCO
3
,
the color of the sample may develop only partially, or may rapidly fade. To resolve this, neutralize the
sample with diluted HCl or NaOH.
Total Chlorine
