YSI 95 User Manual
Page 16
YSI Incorporated
Model 95
12
SECTION 5
SECTION 5
SECTION 5
SECTION 5
PRINCIPLES OF O
PRINCIPLES OF O
PRINCIPLES OF O
PRINCIPLES OF OPERATION
PERATION
PERATION
PERATION
5.1
5.1
5.1
5.1 MEA CLARK OXYGEN SENSOR
MEA CLARK OXYGEN SENSOR
MEA CLARK OXYGEN SENSOR
MEA CLARK OXYGEN SENSOR
The MEA (microelectrrode array) is a steady-state Clark type polarographic (voltammetric)
dissolved oxygen sensor. The sensor is made of a silver anode and a gold cathode (consisting of 100
very small electrodes, each measuring approximately 8 micrometers in diameter) and is separated
from the measured medium by a semi-permeable Teflon membrane. The small dimensions of each
individual micro surface consume a very small amount of oxygen. Large spacing between adjacent
microsurfaces allows for minimal overlap of diffusion layers from adjacent cathode surfaces. This
design produces the minimal stirring dependence of the MEA probe. The temperature sensing
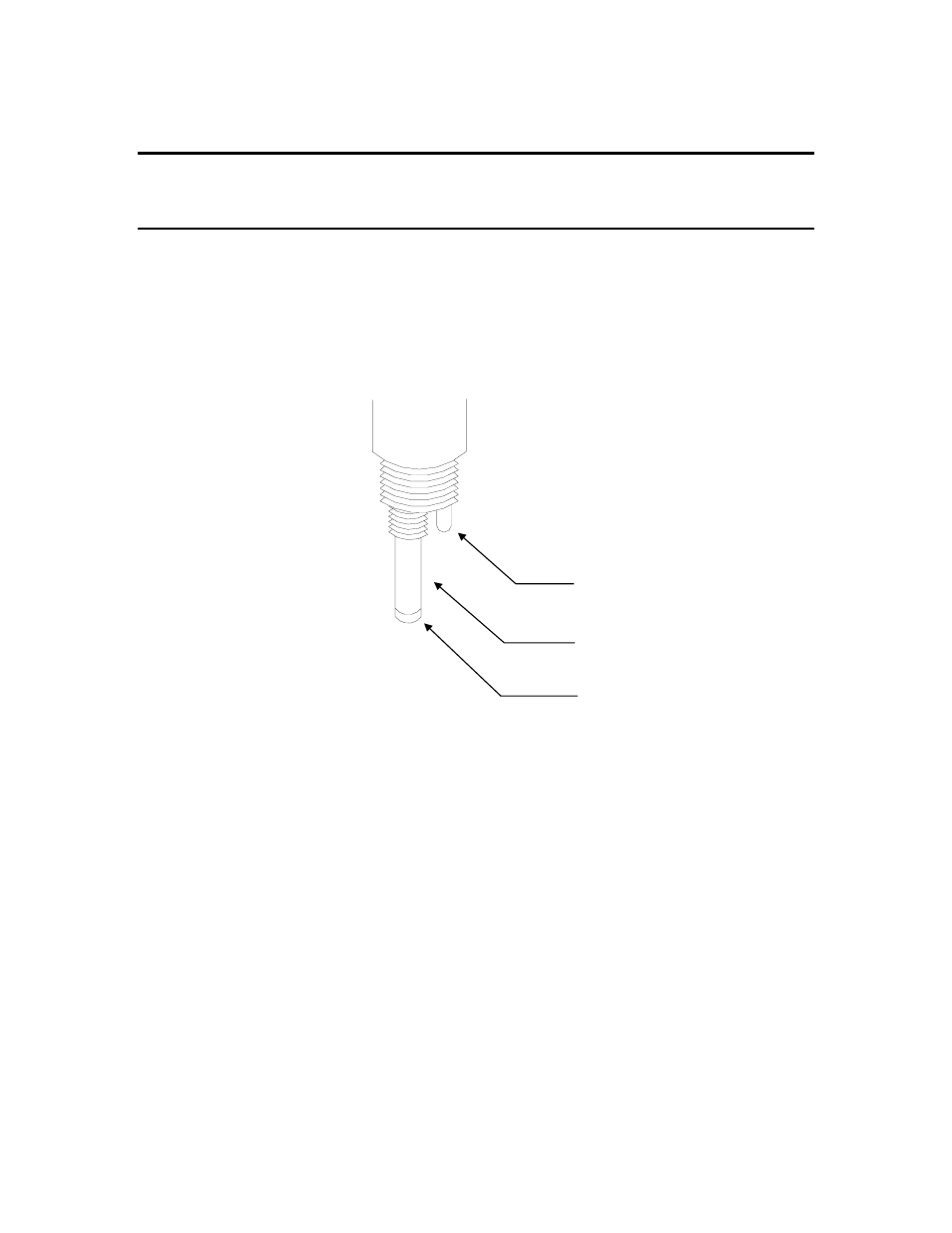
element (thermistor assembly) is mounted next to the oxygen sensor vertically (see Figure 1),
providing temperature readings for the DO system.
The membrane selectively allows oxygen to permeate into the sensor, but prevents most interfering
molecules and fouling materials from entering. Upon permeating through the membrane, oxygen is
reduced at the gold cathode. The current resulting from this reduction is diffusion-limited and is
proportional to the partial pressure of oxygen in the sample. The counter reaction is the oxidation of
silver at the anode/reference electrode that completes the overall electrolytic reaction in the chloride
medium (KCl electrolyte) behind the membrane. These reactions, at the cathode and the anode, are
as follows:
Cathode reaction:
O
2
+ 2H
2
O + 4e
-
==> 4OH
-
Anode reaction:
Ag + Cl
-
==> AgCl + e
-
Figure 5
Temperature sensor
Anode (silver)
MEA Cathode (gold)
