Cell-balancing foundation – Cypress CY8C29x66 User Manual
Page 2
AN2309
November 25, 2007
Document No. 001-17394 Rev. *B
- 2 -
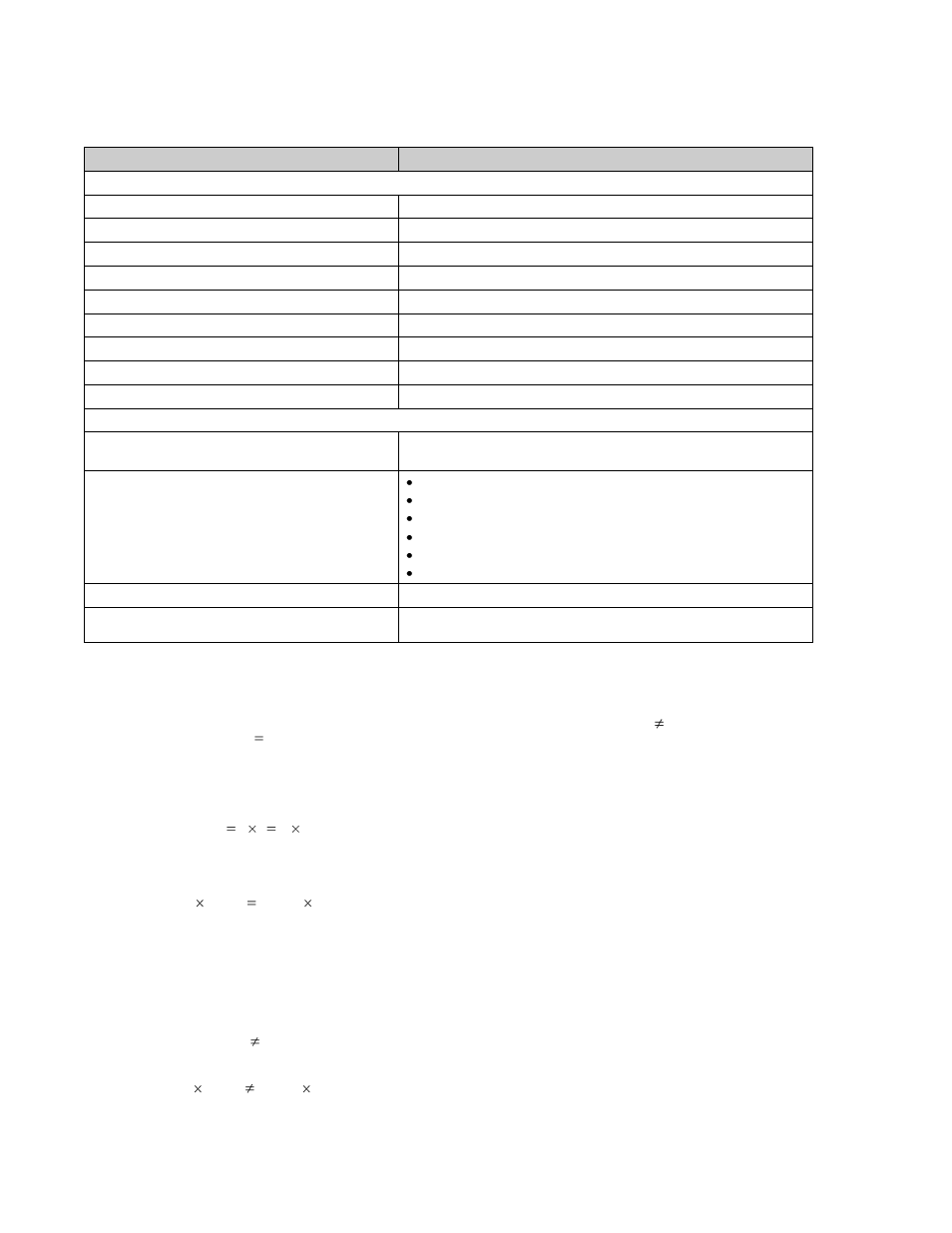
Table 1. Specifications for Two-Cell Li-Ion/Li-Pol Battery Charger with Cell-Balancing Support
Item
Item Value
Battery Charger Parameters
Built-In Battery Charger Type
Two-cell Li-Ion/Li-Pol battery charger
Power Supply Voltage
10…14V
Power Consumption
35 mA
Battery Current Measurement Error (Not Calibrated)
5 percent
Battery Voltage Measurement Error (After Calibration)
0.5 percent
Battery Thermistor Resistance Measurement Error
5 percent
User Interface
2 LEDs
PC Communication Interface
RS232
PC Communication Speed
115200
Cell-Balancing Parameters
Cell-Balancing Algorithms
1. During charge phase
2. During discharge phase
Cell-Balancing Configuration Parameters
Cell-balance circuit resistors nominal
Cell-balance interval parameter
Minimum cell-balance parameter for charge phase
Minimum cell-balance parameter for discharge phase
Minimum charge current value when cell balancing is allowed
VMID value for discharge phase (voltage of middle charged state)
Minimum Cell Balancing During Charge Phase
Equal to the voltage measurement error value (15 mV-30 mV)
Minimum Cell Balancing During Discharge Phase
Equal to the voltage measurement error value (15 mV-30 mV) plus the
internal impedance error (10 mV-30 mV)
Cell-Balancing Foundation
This section describes the fundamentals of cell-balancing
techniques. Cells are considered balanced when:
1
2
Q
Q
cell
cell
Equation 1
The value
cellN
Q
is the charge of cell N. The equation for
the charge is:
Q
I t
C V
Equation 2
Therefore,
can be transformed into the following
equation:
1
1
2
2
C
V
C
V
cell
cell
cell
cell
Equation 3
The value
V
cellN
is the electrochemical potential of the fully
charged cell. The
V
cellN
potential is fixed for a given set of
electrodes is fixed and does not change from cell to cell.
When two cells are unbalanced, the following is true:
1
2
Q
Q
cell
cell
Equation 4
1
1
2
2
C
V
C
V
cell
cell
cell
cell
Equation 5
However,
V
cell
does not change from cell to cell.
Therefore, the cells are unbalanced if:
1
2
C
C
cell
cell
Equation 6
shows two cells that have different capacities,
which is one cause of cell imbalance. A difference in cell-
charge levels, which can be identified by using
is the second cause of cell imbalance. For both kinds of
mismatches in the battery pack
– different cell capacities
and difference cell charge levels
– the highest voltage cell
shows relative charge redundancy and must be shunted
during the charging/discharging process. This is the heart of
the cell-balancing issue.
The main reasons for variation in cell capacity are:
Variations in cell assembly. Today’s factory
manufacturing of cells produces Li-Ion battery backs
with cell capacity matched to three percent.
Different rates in cell degradation. The self-degradation
rate is 30 percent at 500 cycles, which equals 0.06
percent per cycle. But individual cells degrade
differently depending on temperature, charge voltage,
and the particular self- degradation process. For
example, a cell with a lower capacity is exposed to a
higher charge voltage, which degrades it faster, further
reducing its capacity and increasing the pack
imbalance.
