Incorrect placement, Correct placement, Indications and usage – Bausch & Lomb LACRISERT (hydroxypropyl cellulose ophthalmic insert) User Manual
Page 2: Important safety information
US/LAC/14/0004
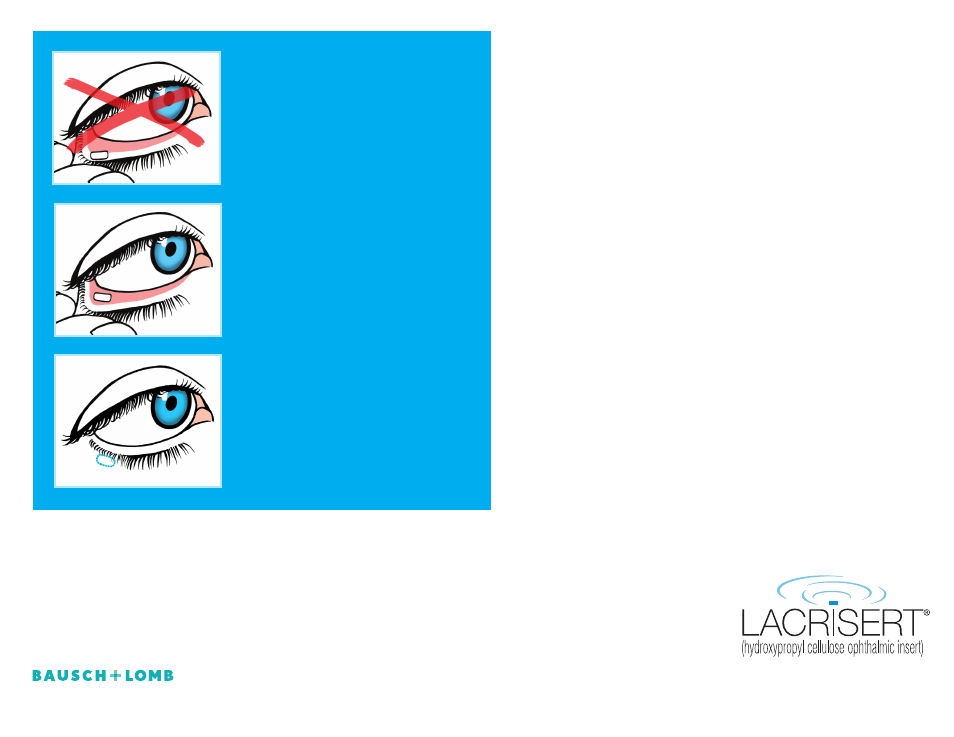
Incorrect Placement
LACRISERT
®
shouldn’t touch the
white part of the eye or be visible
on the rim of the lower lid.
Correct Placement
LACRISERT
®
should rest in the
pink part under the lower eyelid.
References: 1. Lamberts DW, Langston DP, Chu W. A clinical study of slow-releasing artificial tears.
Ophthalmology. 1978;85(8):794-800. 2. LACRISERT package insert [Aton Pharma, Madison NJ].
3. Katz IM, Blackman WM. A soluble-sustained release ophthalmic delivery unit. Am J Ophthalmol.
1977;5(8):728-734. 4. Breslin CW, Katz J, Kaufman HE, Katz I. Slow release artificial tears. In:Leopold
IH, Burns RF, eds. Symposium on ocular therapy. New York: Wiley, 1977;10:77-83. 5. Hovding G,
Aasved H. Slow-release artificial tears (SRAT) in dry eye disease. Acta Ophthalmol (Kbh). 1981;59:842-6.
Please see Prescribing Information that accompanies your prescription.
When placed correctly, it should
be completely out of sight and
feel comfortable. LACRISERT
®
acts like a slow release artificial
tear to help provide ongoing
lubrication and protection.
Once a Day*
2
Slow-release Artificial Tear
1,3-5
for Continuous Lubrication
* In most patients, one LACRISERT
®
placed into each eye, once daily, is effective in providing
all-day symptom relief. Some patients may require twice-daily use for optimal results.
Indications and Usage
LACRISERT
®
(hydroxypropyl cellulose ophthalmic insert)
is indicated in patients with moderate to severe dry eye
syndromes, including keratoconjunctivitis sicca. LACRISERT
®
is indicated especially in patients who remain symptomatic
after an adequate trial of therapy with artificial tear
solutions. LACRISERT
®
is also indicated for patients with
exposure keratitis, decreased corneal sensitivity, and
recurrent corneal erosions.
Important Safety Information
•
LACRISERT
®
(hydroxypropyl cellulose ophthalmic insert)
is contraindicated in patients who are hypersensitive to
hydroxypropyl cellulose.
•
Instructions for inserting and removing LACRISERT
®
should be carefully followed.
•
If improperly placed, LACRISERT
®
may result in corneal
abrasion. Because LACRISERT
®
may cause transient
blurred vision, patients should be instructed to exercise
caution when driving or operating machinery.
•
The following adverse reactions have been reported,
but were in most instances mild and temporary: transient
blurring of vision, ocular discomfort or irritation, matting
or stickiness of eyelashes, photophobia, hypersensitivity,
eyelid edema, and hyperemia.
You are encouraged to report side effects of prescription
drugs to the FDA. Visit
www.FDA.gov/medwatch
or call
1-800-FDA-1088.
Please see accompanying full Prescribing Information,
including Instructions for Use.
Lacrisert is a trademark of Valeant Pharmaceuticals International or its affiliates.
Bausch + Lomb is a trademark of Bausch & Lomb Incorporated or its affiliates.
©2014 Bausch & Lomb Incorporated.
For more information
on LACRISERT
®
, plus
FREE samples and
coupons, go to
www.LACRISERT.com
