Bausch & Lomb PureVision2 for Astigmatism Contact Lenses User Manual
Toric, Package insert / fitting guide
IMPORTANT
This package insert and fitting guide has been developed to provide practitioners
with information covering characteristics of the Bausch + Lomb PureVision
®
2 Toric
(balafilcon A) Visibility Tinted Contact Lens and to illustrate fitting procedures. It
is effective as of June 2013 and supersedes all prior fitting guides for the product
described. Please read carefully and keep this information for future use.
This package insert and fitting guide is intended for the eye care professional, but
should be made available to patients upon request. The eye care professional
should provide the patient with the patient instructions that pertain to the patient’s
prescribed lens and the recommended wearing schedule.
© Bausch & Lomb Incorporated. All rights reserved worldwide.
®/TM are trademarks of Bausch & Lomb Incorporated.
Other product/brand names are trademarks of their respective owners.
Name and Address of Manufacturer:
Bausch & Lomb Incorporated
Rochester, New York, USA 14609
Printed in the U.S.A.
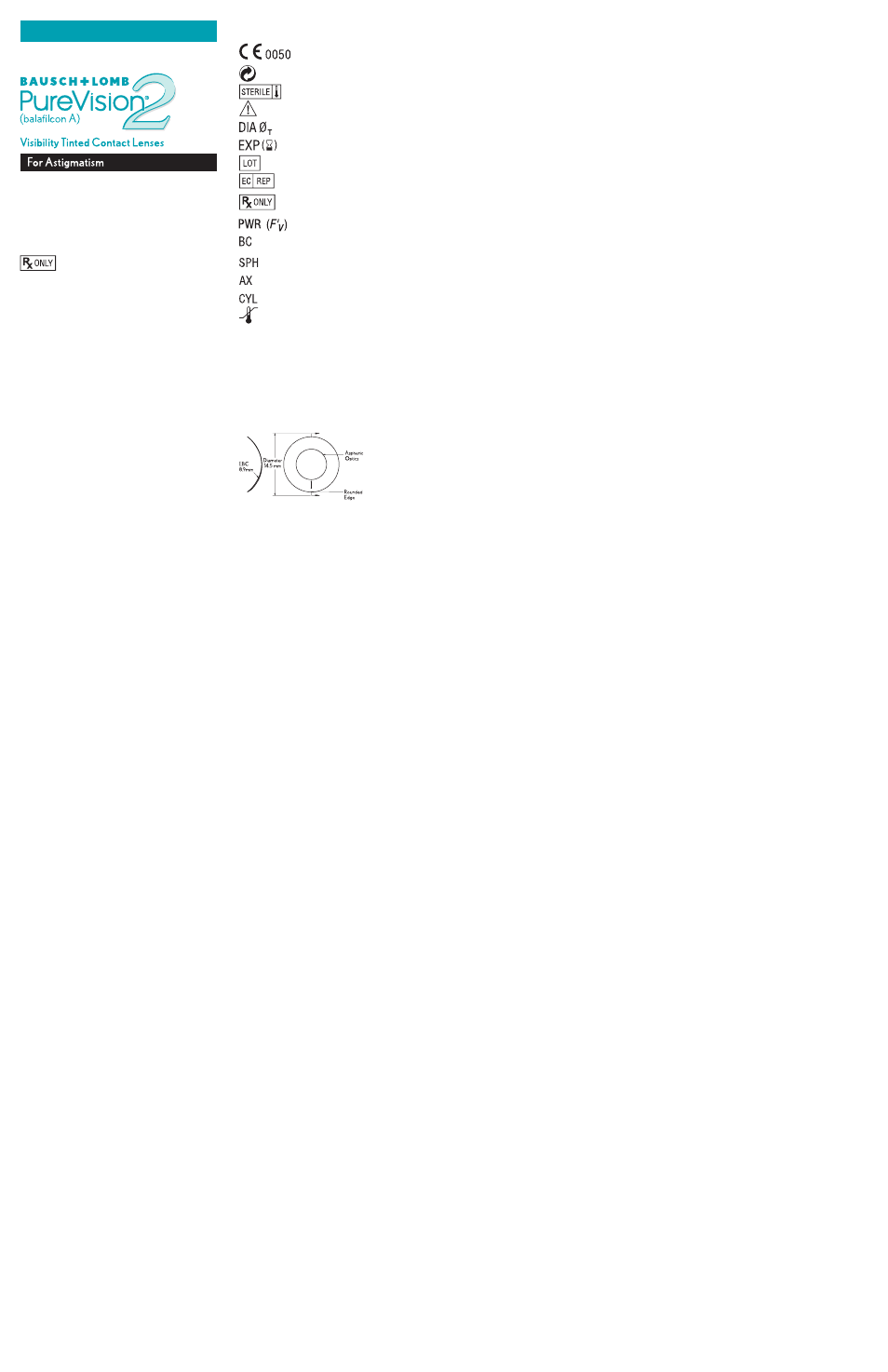
SyMbOl RefeReNce GuIde
For labels and cartons:
Indicates the CE Conformity Marking and the
Notified Body Number
Fee Paid for Waste Management
Sterile Using Steam or Dry Heat
See Instruction Leaflet
Diameter
Use by Date (Expiration Date)
Batch Code
Authorized Representative in European Community
Caution: Federal law restricts this device to sale by or
on the order of a licensed practitioner.
Diopter (Lens Power)
Base Curve
Sphere Power (Diopters)
Cylinder Axis (Degrees)
Cylinder Power (Diopters)
Storage Temperature
TAble Of cONTeNTS
Important 2
Description 3
Lens Parameters Available
5
How the Lens Works (Actions)
5
Indications 6
Contraindications 7
Warnings 8
Precautions 10
Adverse Reactions
14
Clinical Study
15
Selection of Patients
20
Fitting Procedure
21
Pre-fitting Examination
21
Initial Lens Power Selection
21
Initial Lens Evaluation
21
Criteria of a Well-fitted Lens
22
Characteristics of a Tight (Steep) Lens
22
Characteristics of a Loose (Flat) Lens
23
Follow-up Care
23
Practitioner Fitting Sets
24
Wearing Schedule
24
Monovision Fitting Guidelines
25
Patient Selection
25
Eye Selection
25
Special Fitting Considerations
26
Near Add Determination
26
Trial Lens Fitting
27
Adaptation
27
Other Suggestions
28
Handling of Lens
29
Patient Lens Care Directions
29
Frequent Replacement and Disposable Wear
29
Care for a Sticking (Nonmoving) Lens
29
Emergencies 30
Reporting of Adverse Reactions
30
How Supplied
30
deScRIPTION
The Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact
Lens is a soft hydrophilic contact lens which is available as a flexible shell with a toric
surface. The lens material, balafilcon A, is a copolymer of a silicone vinyl carbamate,
N-vinyl-pyrrolidone, a siloxane crosslinker and a vinyl alanine wetting monomer, and
is 36% water by weight when immersed in a sterile borate buffered saline solution.
This lens is tinted blue with up to 300 ppm of Reactive Blue Dye 246.
The physical / optical properties of the lens are:
Specific Gravity:
1.064
Refractive Index:
1.426
Light Transmittance:
C.I.E. value—at least 95%
Water Content:
36%
Oxygen Permeability:
91 x 10
–11
[cm
3
O
2
(STP) x cm]/(sec x cm
2
x mmHg)
@ 35° C Polarographic Method
(Boundary and Edge Corrected)
101 x 10
–11
[cm
3
O
2
(STP) x cm]/(sec x cm
2
x
mmHg) @ 35°C Polarographic Method
(Boundary Corrected, Non-Edge Corrected)
The Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact
Lens, with AerGel™ technology lens material, are manufactured by a cast molding
process and are treated by the Performa™ surface treatment process which
transforms hydrophobic silicone to hydrophilic silicate. The anterior surface of the
lens contains the spherical power, prism ballast and comfort chamfer feature of the
Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact Lens. The
posterior surface is manufactured with a spherocylindrical curve to accommodate
the required astigmatic power.
Aspheric optical surfaces
designed to reduce the
population average spherical
aberration across all sphere,
cylinder, and axis combinations.
A hybrid ballasting geometry
designed to optimize thickness
from apex to base of lens and
offer excellent orientation and
alignment.
A smooth rounded profile
designed to provide comfort
plus optimal movement over
the conjunctival tissue.
(6 o’clock)
Center Guide
Guide Mark System
Each Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact
Lens is marked with 1 Guide Mark, in the lens perimeter at 6 o’clock. This Guide
Mark gives an instant reference for estimating lens rotation and orientation. It is in
effect, a protractor guide on the lens surface. The guide mark make proper axis
orientation and fitting faster and easier.
leNS PARAMeTeRS AVAIlAble
The Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact Lens
is a hemispherical shell of the following dimensions:
Diameter: 14.5mm
Center Thickness:
0.05mm to 0.50mm
Base Curve:
8.9mm
Sphere Powers:
+6.00D to -9.00D in 0.25D steps
(0.50D steps above –6.00D)
Cylinder Powers:
–0.75D, –1.25D, –1.75D, -2.25D and -2.75D
Axis:
10° to 180° in 10° increments
*Additional powers may be introduced over time, check for product availability.
Lens Prism:
Prism is located at the base of the lens to stabilize
lens positioning when lens is on the eye.
Comfort Chamfer:
A wedge-shaped tapered section on the anterior
surface of the lens in the periphery of the lens from
the 3 to 9 o’clock areas. This reduces lens thickness.
HOW THe leNS WORKS (AcTIONS)
In its hydrated state, the Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility
Tinted Contact Lens has a unique hybrid ballasting design that results in excellent
stability and when placed on the cornea, acts as a refracting medium to focus light
rays on the retina.
INdIcATIONS
Vision Correction
The Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact Lens
is indicated for daily wear or extended wear from 1 to 30 days between removals,
for cleaning and disinfection or disposal of the lens, as recommended by the eye
care professional. The lens is indicated for the correction of refractive ametropia
(myopia, hyperopia and astigmatism) in aphakic and/or not-aphakic persons with
non-diseased eyes, exhibiting astigmatism of up to 5.00 diopters, that does not
interfere with visual acuity. The lens may be prescribed for Frequent / Planned
Replacement Wear or Disposable Wear in spherical powers ranging from +6.00D
to -9.00D when prescribed for up to 30 days of extended wear and from +20.00D
to –20.00D for daily wear or extended wear up to 7 days.
Note: See the WARNINGS reference to the relationship between lens wearing
schedule and corneal complications.
FREQUENT / PLANNED REPLACEMENT WEAR
When prescribed for Frequent / Planned Replacement Wear, the Bausch + Lomb
PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact Lens is to be cleaned,
rinsed and disinfected each time it is removed from the patient’s eye and discarded
after the recommended wearing period prescribed by the eye care professional.
The lens may be disinfected using a chemical disinfection system.
DISPOSABLE WEAR
When prescribed for Disposable Wear, the Bausch + Lomb PureVision
®
2 Toric
(balafilcon A) Visibility Tinted Contact Lens is to be discarded after each removal.
cONTRAINdIcATIONS
(ReASONS NOT TO uSe)
DO NOT USE the Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility
Tinted Contact Lens when any of the following conditions exist:
• Acute and subacute inflammation or infection of the anterior chamber of
the eye
• Any eye disease, injury, or abnormality that affects the cornea, conjunctiva, or
eyelids
• Severe insufficiency of lacrimal secretion (dry eyes)
• Corneal hypoesthesia (reduced corneal sensitivity)
• Any systemic disease that may affect the eye or be exaggerated by wearing
contact lenses
• Allergic reactions of ocular surfaces or adnexa (surrounding tissue) that
may be induced or exaggerated by wearing contact lenses or use of contact
lens solutions
• Allergy to any ingredient, such as mercury or Thimerosal, in a solution which
is to be used to care for the Bausch + Lomb PureVision
®
2 Toric (balafilcon A)
Visibility Tinted Contact Lens
• Any active corneal infection (bacterial, fungal, or viral)
• If eyes become red or irritated
WARNINGS
After a thorough eye examination, including appropriate medical background,
patients should be fully apprised by the prescribing professional of all the risks
with contact lens wear. Patients should be advised of the following warnings
pertaining to contact lens wear:
• Problems with contact lenses and lens care products could result in serious
injury to the eye. It is essential that patients follow their eye care professional’s
direction and all labeling instructions for proper use of lenses and lens care
products, including the lens case. Eye problems, including corneal ulcers, can
develop rapidly and lead to loss of vision.
• When prescribed for Frequent / Planned Replacement Wear, the need for
strict compliance with the care regimen including cleaning of the lens case,
wearing restrictions, wearing schedule, and follow-up visit schedule should be
emphasized to the patient.
• Studies have shown that contact lens wearers who are smokers have a higher
incidence of adverse reactions than nonsmokers.
• If a patient experiences eye discomfort, excessive tearing, vision changes, or
redness of the eye, the patient should be instructed to immediately remove
lenses and promptly contact his or her eye care professional.
EXTENDED WEAR
• The risk of microbial keratitis has been shown to be greater among users of
extended wear contact lenses than among users of daily wear contact lenses.
The risk among extended wear lens users increases with the number of
consecutive days that the lenses are worn between removals, beginning with the
first overnight use.
• Some researchers believe that these complications are caused by one or
more of the following: a weakening of the cornea’s resistance to infections,
particularly during a closed-eye condition, as a result of hypoxia; an eye
environment which is somewhat more conducive to the growth of bacteria
and other microorganisms, particularly when a regular periodic lens removal
and disinfecting or disposal schedule has not been adhered to by the patient;
improper lens disinfection or cleaning by the patient; contamination of lens
care products; poor personal hygiene by the patient; patient unsuitability to the
particular lens or wearing schedule; accumulation of lens deposits; damage to
the lens; improper fitting; length of wearing time; and the presence of ocular
debris or environmental contaminants.
• While the great majority of patients successfully wear contact lenses, extended
wear of lenses also is reported to be associated with a higher incidence and
degree of epithelial microcysts and infiltrates, and endothelial polymegathism,
which require consideration of discontinuation or restriction of extended wear.
The epithelial conditions are reversible upon discontinuation of extended wear.
• The risk of microbial keratitis has not been determined for this lens. Post
marketing studies are in progress.
• The reversibility of endothelial effects of contact lens wear has not been
conclusively established. As a result, professionals’ views of extended wearing
times vary from not prescribing extended wear at all to prescribing flexible
wearing times from occasional overnight wear to prescribing extended wearing
periods from 1 to 30 days with specified intervals of no lens wear for certain
patients, with follow-up visits, and with proper care regimen.
• If a patient experiences eye discomfort, excessive tearing, vision changes, or
redness of the eye, the patient should be instructed to
immediately remove
lenses and promptly contact his or her eye care professional.
PRecAuTIONS
Precautions for Eye Care Professionals
• Due to the small number of patients enrolled in clinical investigation of lenses, all
refractive powers, design configurations, or lens parameters available in the lens
material are not evaluated in significant numbers. Consequently, when selecting
an appropriate lens design and parameters, the eye care professional should
consider all characteristics of the lens that can affect lens performance and
ocular health, including oxygen permeability, wettability, central and peripheral
thickness, and optic zone diameter.
• The oxygen transmissibility is below the established threshold required to
prevent overnight corneal edema for portions of the power range, including
plus powers and some low minus power lenses.
1
In the U.S. clinical study of the
PureVision (spherical) lens, the rate of infiltrative keratitis was found to be higher
with higher lens powers (see Clinical Study section of the package insert).
• The potential impact of these factors on the patient’s ocular health should be
carefully weighed against the patient’s need for refractive correction; therefore,
the prescribing eye care professional should carefully monitor the continuing
ocular health of the patient and lens performance on eye.
• Eye care professionals should instruct the patient to REMOVE A LENS
IMMEDIATELY if an eye becomes red or irritated.
• Fluorescein, a yellow dye, should not be used while the lenses are on the eyes.
The lenses absorb this dye and become discolored. Whenever fluorescein
is used in eyes, the eyes should be flushed with sterile saline solution that is
recommended for in-eye use.
• The patient should be instructed to always discard disposable lenses and lenses
worn on a Frequent / Planned Replacement schedule after the recommended
wearing schedule prescribed by the eye care professional.
• Some patients will not be able to tolerate continuous wear even if able to
tolerate the same or another lens on a daily wear basis. Some patients who
are able to tolerate continuous wear will not be able to wear their lenses
continuously for 30 days. Patients should be carefully evaluated for continuous
wear prior to prescription and dispensing, and eye care professionals should
conduct early and frequent follow-up examination to determine ocular
response to continuous wear.
• As with any contact lens, follow-up visits are necessary to assure the
continuing health of the patient’s eyes. The patient should be instructed as to a
recommended follow-up schedule.
• Aphakic patients should not be fitted with Bausch + Lomb PureVision
®
2 Toric
(balafilcon A) Visibility Tinted Contact Lenses until the determination is made
that the eye has healed completely.
Eye care professionals should carefully instruct patients about the following lens
care and safety precautions. It is strongly recommended that patients be provided
with a copy of the Bausch + Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted
Contact Lens Patient Information Booklet available from Bausch + Lomb and
understand its contents prior to dispensing the lenses.
Handling Precautions
• Always wash and rinse hands before handling lenses. Do not get cosmetics,
lotions, soaps, creams, deodorants, or sprays in the eyes or on the lenses. It is
best to put on lenses before putting on makeup. Water-base cosmetics are less
likely to damage lenses than oil-base products.
• Be sure that before leaving the eye care professional’s office, the patient is able
to remove lenses promptly or have someone else available to remove them.
• Be certain that the fingers or hands are free of foreign materials before touching
lenses, as microscopic scratches of the lenses may occur, causing distorted
vision and/or injury to the eye.
• Always handle lenses carefully and avoid dropping them.
• Do not touch the lens with fingernails.
• Carefully follow the handling, insertion, removal, cleaning, disinfecting, storing
and wearing instructions in the Patient Information Booklet for the Bausch +
Lomb PureVision
®
2 Toric (balafilcon A) Visibility Tinted Contact Lens and those
prescribed by the eye care professional.
• Never use tweezers or other tools to remove lenses from the lens container
unless specifically indicated for that use. Pour the lens into the hand.
Solution Precautions
Do not use the Allergan Ultracare Disinfecting System or any of its
components (Ultracare Disinfecting Solution, Ultracare Neutralizing
Tablets, Lens Plus Daily Cleaner, and Ultrazyme Enzymatic Cleaner) to
clean and disinfect the Bausch + Lomb PureVision
®
2 Toric (balafilcon A)
Visibility Tinted Contact Lens because the lens dimensions will be altered.
Eye injury due to irritation or infection may result from lens contamination. To reduce
the risk of contamination, review the appropriate manufacturer’s labeled lens care
instructions with the patient.
• Always use
fresh unexpired lens care solutions.
• Always follow directions in the package inserts for the use of contact lens
solutions.
• Sterile unpreserved solutions, when used, should be discarded after the time
specified in the labeling directions.
• Always keep the lenses completely immersed in the recommended storage
solution when lenses are not being worn (stored). Prolonged periods of drying
will damage lenses. Follow the lens care directions for Care for a Dried Out
(Dehydrated) Lens in the Patient Information Booklet if lens surface does
become dried out.
• Do not use saliva or anything other than the recommended solution for
lubricating or wetting lenses.
• Tap water, distilled water or homemade saline should not be used as a substitute
for any component in the lens care regimen since they have been associated
with an Acanthamoeba keratitis infection.
• Never use conventional hard contact lens solutions that are not also
recommended for use with prescribed lenses.
• Do not mix or alternate lens care systems or solutions unless indicated in the
lens care system labeling.
• Do not heat the chemical disinfection solution or lenses.
Lens Wearing Precautions
• Never wear lenses beyond the period recommended by the eye care
professional.
• If the lens sticks (stops moving) on the eye, follow the recommended directions
on Care for a Sticking Lens. The lens should move freely on the eye for the
continued health of the eye. If nonmovement of the lens continues, the patient
should be instructed to
immediately consult his or her eye care professional.
• Avoid, if possible, all harmful or irritating vapors and fumes while wearing lenses.
• If aerosol products are used while wearing lenses, exercise caution and keep
eyes closed until the spray has settled.
Lens Case Precautions
• Contact lens cases can be a source of bacterial growth. To prevent
contamination and to help avoid serious eye injury, always empty and rinse the
lens case with fresh, sterile rinsing solution and allow to air dry.
• Lens cases should be replaced monthly or as frequently as recommended by
the lens case manufacturer or eye care professional.
Topics to Discuss with the Patient
• As with any contact lens, follow-up visits are necessary to assure the continuing
health of the eyes. The patient should be instructed as to a recommended
follow-up schedule.
• Patients should be advised about wearing lenses during sporting and water
related activities. Exposure to water while wearing contact lenses in activities
such as swimming, water skiing and hot tubs may increase the risk of ocular
infection including but not limited to Acanthamoeba keratitis.
• Always contact the eye care professional before using any medicine in the eyes.
Who Should Know That the Patient is Wearing Contact Lenses
• Patients should inform their doctor (health care professional) about being a
contact lens wearer.
• Patients should always inform their employer of being a contact lens wearer.
Some jobs may require the use of eye protection equipment or may require that
you do not wear lenses.
AdVeRSe ReAcTIONS
The patient should be informed that the following problems may occur:
• Eyes stinging, burning, itching (irritation), or other eye pain
• Comfort is less than when lens was first placed on eye
• Abnormal feeling of something in the eye (foreign body, scratched area)
• Excessive watering (tearing) of the eyes
• Unusual eye secretions
• Redness of the eyes
• Reduced sharpness of vision (poor visual acuity)
• Blurred vision, rainbows, or halos around objects
• Sensitivity to light (photophobia)
• Dry eyes
If the patient notices any of the above, he or she should be instructed to
•
Immediately remove the lenses.
• If the discomfort or problem stops, the patient should look closely at the lens. If
the lens is in any way damaged, do not put the lens back on the eye. The patient
should place the lens in the storage case and contact the eye care professional.
If the lens has dirt, an eyelash, or other foreign body on it, or the problem stops
and the lens appears undamaged, the patient should thoroughly clean, rinse,
and disinfect the lenses; then reinsert them. After reinsertion, if the problem
continues, the patient should immediately remove the lenses and consult
his or her eye care professional.
• If the above symptoms continue after removal of the lens, or upon reinsertion
of a lens, or upon insertion of a new lens, the patient should
immediately
remove the lenses and contact his or her eye care professional or
physician, who must determine the need for examination, treatment or referral
without delay. (See Important Treatment Information for Adverse Reactions.)
A serious condition such as infection, corneal ulcer, corneal vascularization, or
iritis may be present, and may progress rapidly. Less serious reactions such as
abrasions, epithelial staining or bacterial conjunctivitis must be managed and
treated carefully to avoid more serious complications.
PACKAGE INSERT / FITTING GUIDE
SL-7345
8129200
2
6
10
14
5
9
13
4
8
12
3
7
11
1
Holden BA, Mertz GW. Critical Oxygen Levels to Avoid Corneal Edema for Daily and Extended Wear
Contact Lenses. Invest Ophthalmol Vis Sci 25:1162, 1984.
CAUTION: Federal law restricts this device to
sale by or on the order of a licensed practitioner.
Toric
