Bausch & Lomb PureVision Multi-Focal Contact Lenses User Manual
Page 5
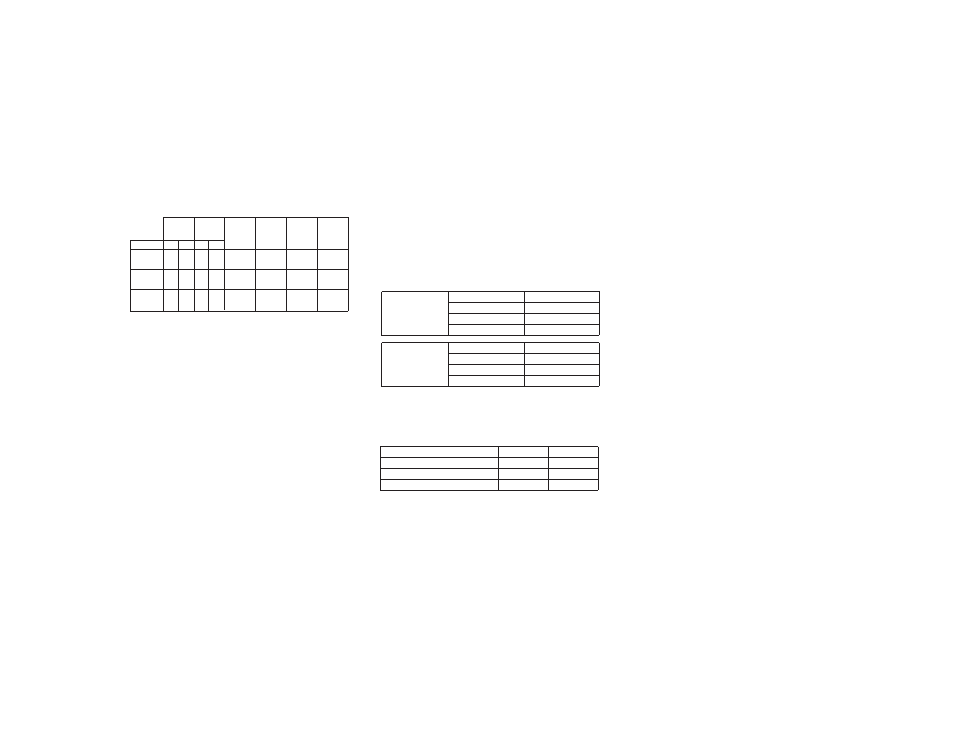
Relative
Risk Upper 95% Clinically
PureVision Control PureVision/ Difference Confidence Significant
Endpoint n % n % Control in % Level Difference
Slit Lamp
Findings
≥
Grade 2 138 17.5% 139 17.6% 1.0
-0.1% 2.6% 10.0%
Corneal
Infiltrates
≥
Grade 2 23 2.9% 10 1.3% 2.3 1.6% 2.9% 5.0%
Visual Acuity
Worse than
20/40 0 0.0% 2 0.3% 0.0 -0.3% 0.1% 5.0%
Summary of Slit Lamp Findings
Slit lamp examinations were conducted at every study visit. Each graded slit
lamp parameter was scored on a qualitative grade scale ranging from 0 to 4,
with Grade 0 representing the absence of findings, and Grades 1 through 4
representing successively worse findings. For each study eye, a determination
was made for each parameter as to whether, or not a positive finding was pre-
sented at any visit. The following table describes slit lamp findings ≥ Grade 2
and ungraded slit lamp findings.
PureVision
Control
Graded Slit Lamp Findings ( ≥ Grade 2)
Any Finding
1,2
17.5%
17.6%
Corneal Staining 8.2% 8.4%
Limbal Injection 3.7% 4.3%
Bulbar Injection 5.2% 4.7%
Tarsal Conjunctival Abnormalities
3.9% 3.9%
Corneal Infiltrates
1
2.9% 1.3%
Epithelial Edema
1.3%
1.4%
Epithelial Microcysts 1.0%
1.0%
Corneal Neovascularization
1.0% 1.7%
Ungraded Slit Lamp Findings
Other Anterior Segment Abnormalities
3
13.2%
13.8%
External Adnexa Abnormalities 2.7%
2.7%
Conjunctivitis
2.4%
2.0%
Corneal Striae
0.0%
0.3%
1/ Slit Lamp Finding and Corneal Infiltrates
≥
Grade 2 were the safety endpoints for
this study.
2/ The total of all Graded slit lamp findings does not equal the category of Any Finding.
3/ The more common findings identified as Other Anterior Segment Abnormalities included:
conjunctival staining; dimple veils; mucin balls; lipid deposits; and ghost vessels.
It should be noted that the PureVision Contact Lens and the Control lens were
each fit on only the right or left eye for each subject. Rates per
subject are expected to be higher when lenses are fit on both eyes.
Corneal Infiltrates
The following table describes the rate of corneal infiltrates according to the
lens power used.
Corneal Infiltrates
Lens Power
( ≥ Grade 2)
PureVision Plano to –3.00 1.7%
-3.25 to –6.00
3.2%
>-6.00 6.4%
Total 2.9%
Control Plano to –3.00 0.9%
-3.25 to –6.00 1.5%
>-6.00 1.3%
Total 1.3%
Other Lens-Related Adverse Events
In addition to the outcomes described above, the following lens related
adverse events were noted. This table does not include conjunctivitis or tarsal
conjunctival abnormalities, e.g., giant papillary conjunctivitis.
Other Important Lens-Related Adverse Events
PureVision Control
Corneal Scar 14 (1.8%) 5 (0.6%)
Other Ocular Inflammation* 10 (1.3%) 2 (0.3%)
Anterior Chamber Reaction 2 (0.3%) 1 (0.1%)
Permanent Loss of Vision
0 (0.0%) 0 (0.0%)
*Other Ocular Inflammation includes episcleritis, scleritis, iritis/uveitis.
This condition was reported in association with other conditions such as
keratitis, corneal infiltrates, blepharitis, corneal abrasion, and contact lens
over wear.
It should be noted that the PureVision Contact Lens and Control lenses were
each fit on only the right or left eye for each subject. Rates per
subject are expected to be higher when lenses are fit on both eyes.
10
11
Efficacy Outcomes
The contact lens visual acuity was measured at each scheduled and unsched-
uled follow-up visit throughout the one-year study. For the 610 subjects that
completed the study, visual acuity of 20/20 or better was reported for 87%
and 86% of the measurements for the PureVision Contact Lens and Control
lens, respectively. Similarly, visual acuity of 20/25 or better was reported
98% and 97% of the times for the PureVision Contact Lens and Control lens.
Wearing Time
In this U.S. clinical study subjects were required to maintain a minimum
wearing time in order to continue in the study. For the subjects that com-
pleted the study, the average continuous wear time for the PureVision
Contact Lens was at least 28.0 days per month, from the 2-Month visit
through the 12-Month visit. At these visits the same subjects reported they
were able to wear the PureVision Contact Lens at least 22 days continuously
94% of the times they were asked.
During the course of the study, 15 subjects were discontinued from the study
because they were not able to wear the PureVision Contact Lens for 30 days.
Twenty-one (21) subjects were discontinued from the study because they
were not able to wear the Control lens for 7 days.
Overnight Corneal Swelling
Two separate studies with the PureVision Lens (spherical) assessed the
corneal swelling response induced by overnight contact lens wear. In the
first study, 30 subjects each wore either a +3.00D, -3.00D, or –9.00D
PureVision Contact Lens and an equivalent power lens made from a conven-
tional hydrogel material (Control lens) on the contralateral eye overnight
under closed eye conditions for approximately eight hours. The corneal
swelling, measured as the percent increase in the center thickness of the
cornea, with the Control lens (9.1%) was significantly greater than that
measured in conjunction with the PureVision Contact Lenses (4.1%). In the
second study, the corneal swelling response was measured under similar
conditions. In this study the response to a –3.00D PureVision Contact Lens
(3.0%) was compared to the swelling response to no lens wear (1.9%). The
responses were not statistically different (p-value > 0.05).
SELECTION OF PATIENTS:
The eye care professional should not fit patients who cannot or will not
adhere to a recommended care or replacement regimen, or are unable to
place and remove the lenses should not be provided with them. Failure to
follow handling and cleaning instructions could lead to serious eye infections
which might result in corneal ulcers.
Patient communication is vital because it relates not only to patient selec-
tion but also to ensure compliance. It is also necessary to discuss the infor-
mation contained in the Patient Information Booklet with the patient at the
time of the initial examination.
Patients selected to wear PureVision Multi-Focal Contact Lenses should be
chosen for their motivation to wear contact lenses, general health and coop-
12
