C.B.S. Scientific EBU-222 User Manual
Page 9
EBU-222 Instruction Manual, version 7/28/2011
9
www.cbsscientific.com
SECTION 4
APPLICATIONS & RUNNING CONDITIONS FOR TANK TYPE ELECTRO-BLOTTING
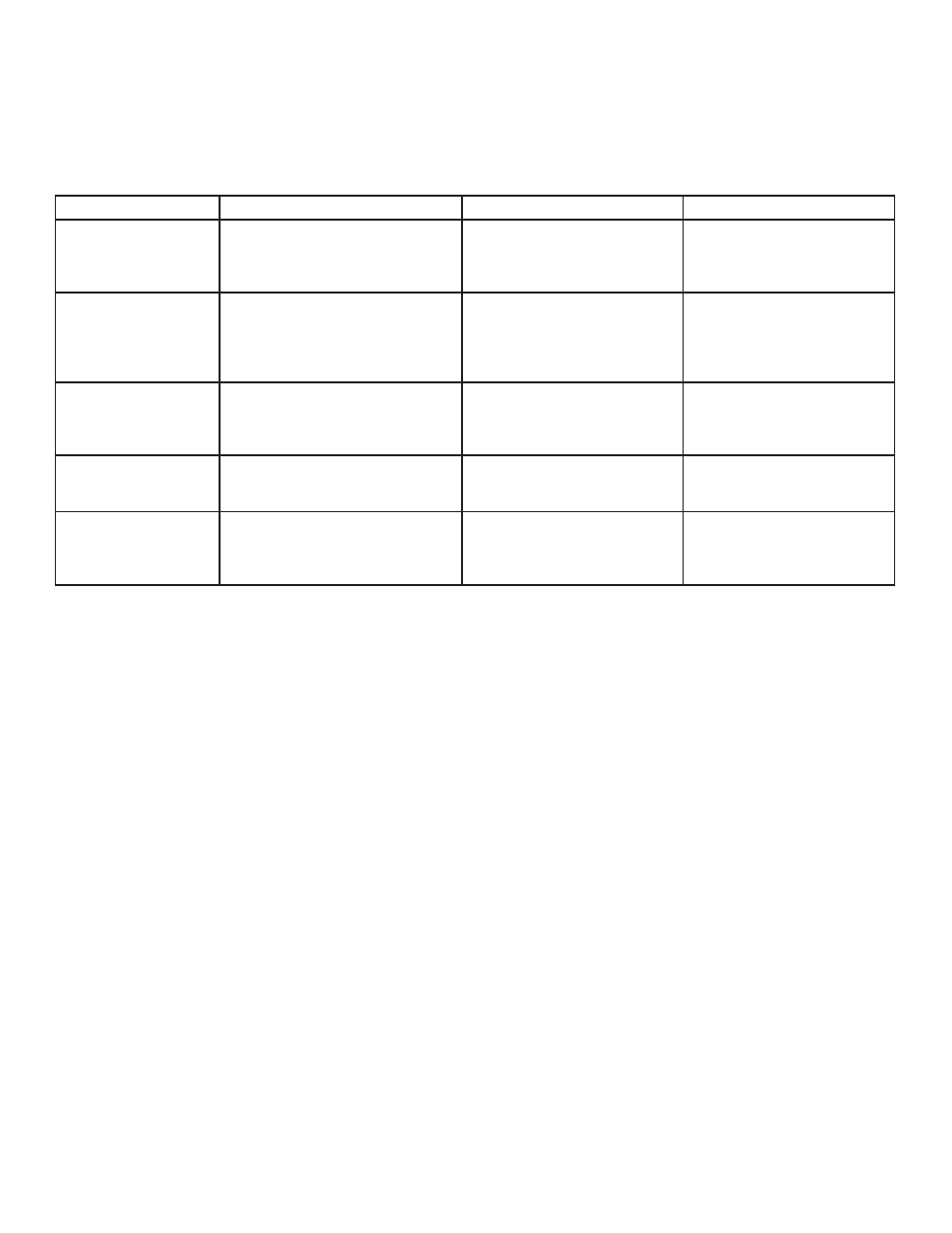
4.1 RECOMMENDED BUFFERS, MEMBRANES, POWER SETTINGS AND TRANSFER TIMES
Gel Type
Blotting Buffer
Transfer Membrane
Power
denaturing: SDS-PAGE
(Western)
Towbin (25mM Tris/192mM Glycine/ pH 8.3/
Methanol 20% (w/v)
nitrocellulose/nylon PVDF: ion-exchange • room temperature, 70V-100V,
200mA, 1.5- 4 hours
• 4ºC, 150V, 400mA, .5-2 hours
• Overnight, 30V, 75mA
non-denaturing: SDS-PAGE
(acidic or neutral proteins)
25mMTris/192mM Glycine, pH 8.3
25mM sodium phosphate, pH6.5
15mM sodium borate, pH9.2
nitrocellulose; nylon PVDF; ion-
exchange
diazo-paper
diazo-paper
• room temperature, 60V, 200mA, 4
hours
• 4ºC, 125V, 400mA, 1.5 hours
• Overnight, 30V, 100mA
IEF (native-basic proteins,
acid urea gels)
0.7% acetic acid (w/v)*
nitrocellulose; nylon
diazo-paper
• room temperature, 70V, 400mA, 4
hours
• 4ºC, 100V, 600mA, 1.5 hours
• Overnight, 30V, 200mA
DNA
(Southern)
1XTAE or agarose
0.5X TBE, acrylamide
nylon; +charged
nylon, neutral or +charged
• room temperature, 30-40V, 25-
150mA, 2-4 hours
• 4ºC, 125V, 400mA, 1.5 hours
RNA
(Northern)
19.6mM phosphate/5.4mM citrate
pH 3.0 or 25mM sodium phosphate pH 6.5
nylon nitrocellulose
• room temperature, 60V, 200mA, 4
hours
• 4ºC, 125V, 400mA, 1.5 hours
• Overnight, 30V, 200mA
4.2 REFERENCES
1). Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K. (ed.) (1993).
Current Protocols in Molecular Biology. Vol. 2, Greene Publishing Associates, Inc. and John Wiley & Sons,
Inc., Ch.10.
2). Burnette, W.N. (1981). Western blotting: Electrophoretic transfer of proteins from sodium dodecyl sulfate-
polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodin-
ated protein A. Anal. Biochem. 112:195-203.
3). Peluso, R.W. and Rosenberg, G.H. (1987). Quantitative electrotransfer of proteins from sodium dodecyl
sulfate polyacrylamide gels onto positively charged nylon membranes. Anal. Biochem. 162:389-398.
4). Perides, G. Plagens, U., and Traub, P. (1986). Protein transfer from fixed, stained and dried polyacryl-
amide gels and immunoblot with protein A-gold. Anal. Biochem. 152:94-99.
5). Tesfaigzi, J., Smith-Harrison, W., and Carlson, D.M. (1994). A simple method for reusing western blots on
PVDF membranes. Biotechniques. 17:268-269.
6). Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide
gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76: 4350-
4354.
7). Sambrook, J., Fritsch, E.F., Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual. 2nd ed. Cold
Spring Harbor Laboratory Press, Cold Spring Harbor, New York. 18.47-18.61.
