UEi Test Instruments SMART BELL User Manual
Page 12
P
erfeCt
C
omBuStioN
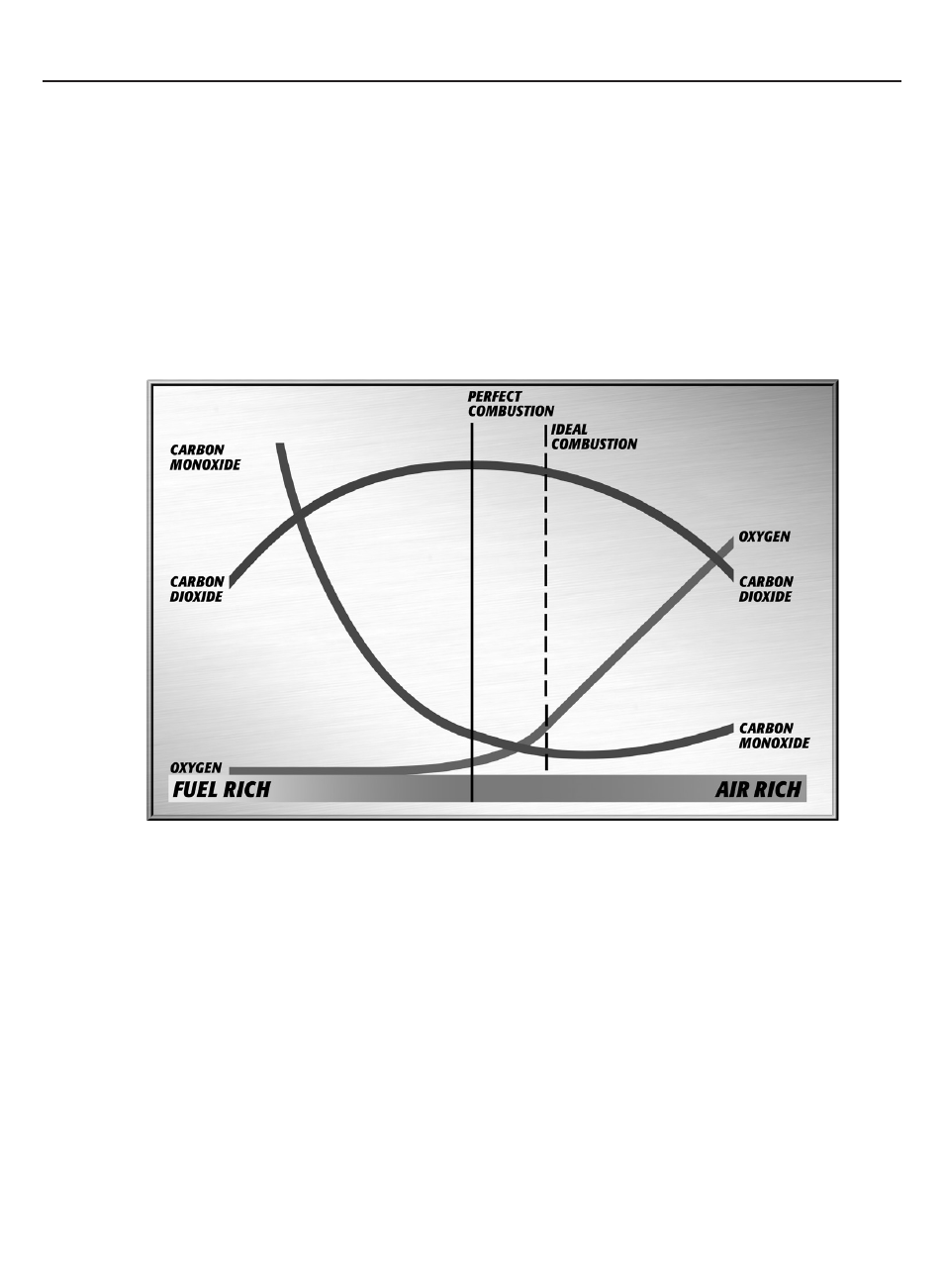
The term perfect combustion is also called stoichiometric combustion. This is the point where all of the fuel
is burned with all the oxygen, leaving no undesirable byproducts. At this point all of the hydrogen in the fuel (H2) would
combine with oxygen to form H2O, all of the carbon (C) would combine to form CO2, and all of the sulfur (S) would
form SO2. There would be no additional air to carry heat away from the fire, and no undesirable byproducts would be
created. In practice this isn’t possible due to the inability to completely mix the fuel and air, so an additional amount of air
is used to completely burn the fuel.
The chart in figure 4 illustrates the relationship between the main flue gas components that provide an indication of the
performance of the combustion process.
As you move left to right you are going from a rich to lean condition. The term Air rich is equivalent to fuel lean, and
simply indicates a situation where the excess air is much higher.
To adjust the combustion process you are given the best overall picture of the condition by measuring all three parameters.
Each of the parameters performs differently as you move through the adjustment of a combustion process
CO2 – This is the gas that was most commonly used for adjusting combustion equipment. A tool called
an Orstat, or wet chemical kit would give you a snapshot of the CO2 value. As you can see by the graph,
CO2 is maximized when the process is running at perfect combustion. Because this isn’t possible, the goal
has always been to maximize CO2. The trouble is that this can occur at two places in the graph, once on
the fuel rich side, and once on the fuel lean side. A smoke test is used to first place you on the
right side of the graph, and then CO2 was maximized to reach the highest value possible.
2. O2 is the next gas that is measured. At perfect combustion all of the O2 in the atmosphere is consume
so
very
little
remains in the flue gases. If you adjust with this gas you are more certain to be on the correct
side of perfect combustion, but you may still be creating carbon monoxide (CO) due to insufficient levels of
O2 to completely burn the carbon in the fuel. This may lead to sooty buildup, reducing efficiency, but you
are also not extracting all of the energy the fuel has to offer.
3. Carbon Monoxide (CO) is the last gas listed. As you can see on the left side of the chart CO production
is the highest. At ideal combustion this level is the lowest possible, but if the other gases are not available
you may be adding too much excess air leading to losses in efficiency. Also if the amount of air being fed to
the combustion process is increased too high it may start cooling the combustion chamber down and begin
creating CO. Once you are at this point without measuring O2 or CO2, you may instinctively add more
O2 to reduce the CO level and end up creating more.
figure 4
10
