UEi Test Instruments SMART BELL User Manual
Page 11
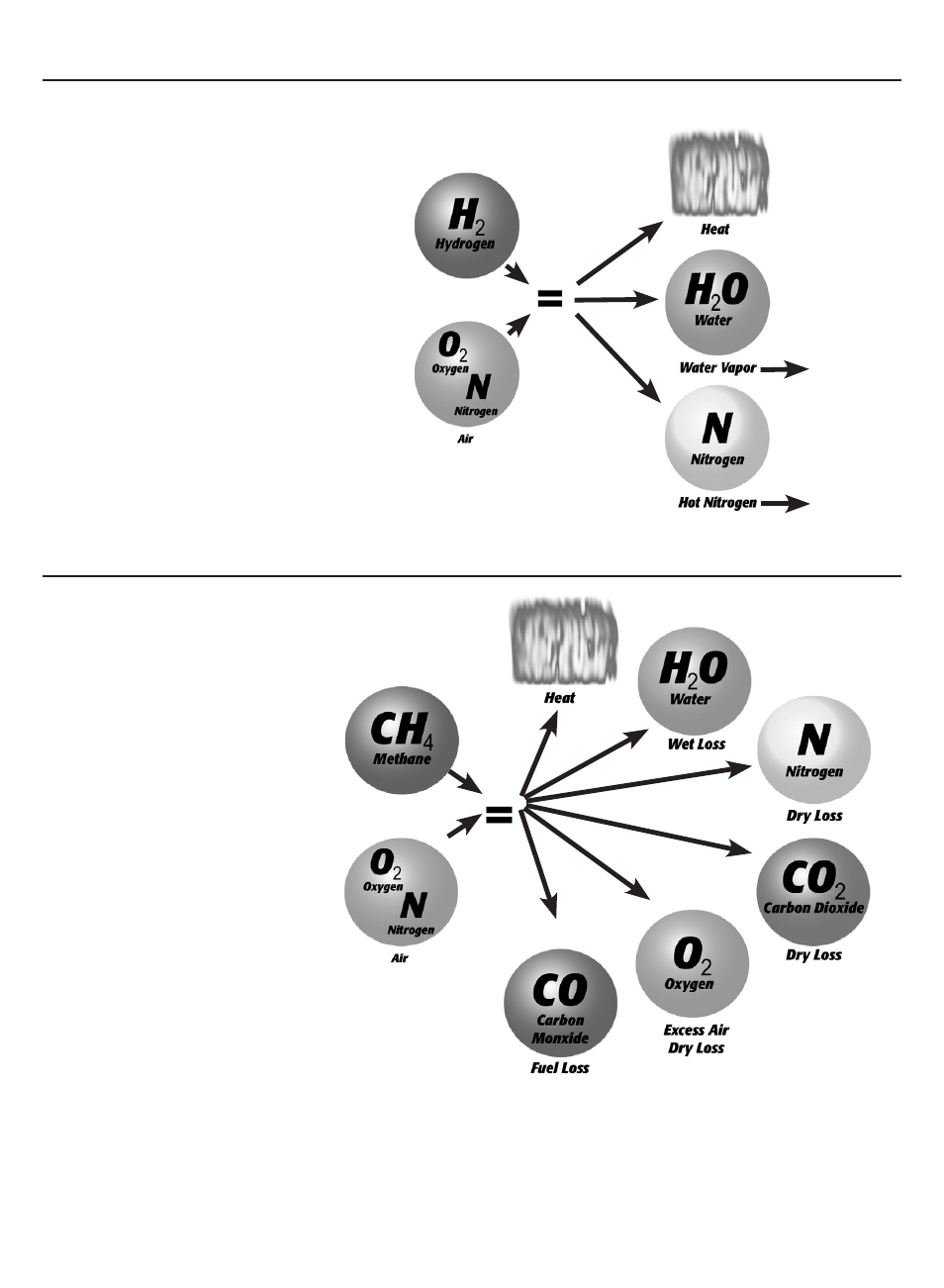
N
ear
i
deal
C
omBuStioN
This is When we burn pure hydro-
gen in the air. Our atmosphere is 20.9% oxy-
gen with the remaining 79.1% nitrogen.
This is nearly as desirable as the example for
ideal combustion with the only added loss
being the heat that is carried away from your
target with the nitrogen. Because nitrogen isn’t
part of the combustion process, it enters the
combustion chamber at the inlet temperature
and leaves with some of the heat created by the
combustion. If this isn’t recovered at the heat
exchanger it is lost up the flue.
The main problem with this example is again
the availability and cost of pure hydrogen.
B
eSt
o
f
t
he
r
eal
w
orld
Natural gas is a readily avail-
able fuel, and our atmosphere contains
sufficient oxygen. When this is used as a
fuel we get the reaction; shown in figure
3.
Now the other added byproducts are
CO2 and hot nitrogen compared to
the Ideal World situation. In addition
to this we have added the byproduct
Excess Air.
Excess Air is exactly what the name
implies, air that is in excess of what is
needed to burn all of the fuel. The
reason for this is more related to the
ability to mix all of the fuel and O2 for
complete combustion. Without some
amount of excess air not all of the fuel
would burn completely, and this leads to
the formation of CO instead of CO2.
Other fuels all contain the basic ingre-
dients for combustion, but also may
include other components such as
sulfur, fuel bound nitrogen, soot and
ash and water. These either react with
the oxygen to form other pollutants or
contribute to
additional losses.
Wet Loss
Dry Loss
figure 3
Carbon Monoxide
is formed from incomplete combustion (partial oxidation of carbon in
the fuel). Typical causes are incomplete mixing of fuel and air, low combustion temperatures,
or not enough excess air.
9
