Thermo Fisher Scientific Ion Selective Electrodes Iodide User Manual
Page 10
Instruction Manual
Iodide Electrode
10
Temperature Influences
Samples and standards should be at the same temperature since electrode potentials are influenced
by changes in temperature. A 1
o
C difference in temperature results in a 2% error at the 1.0X10
-3
M
concentration level. Because of solubility equilibrium on which the electrode depends, the absolute
potential of the reference electrode changes slowly with the temperature. The slope of the electrode,
as indicated by the factor "S" in the Nernst equation, also varies with temperature. Table 4 gives
values of the "S" factor in the Nernst equation for the iodide ion.
TABLE 4: Temperature vs. Values for the Electrode Slope
Temp (oC)
"S"
0
54.2
10
56.2
20
58.2
25
59.2
30
60.1
40
62.1
50
64.1
If changes in temperature occur, the electrodes should be recalibrated.
The temperature range of the Eutech Iodide Ion Electrodes is 0
o
-80
o
C, provided that temperature
equilibrium has occurred. If temperature varies substantially from room temperature, equilibrium
times up to one hour are recommended.
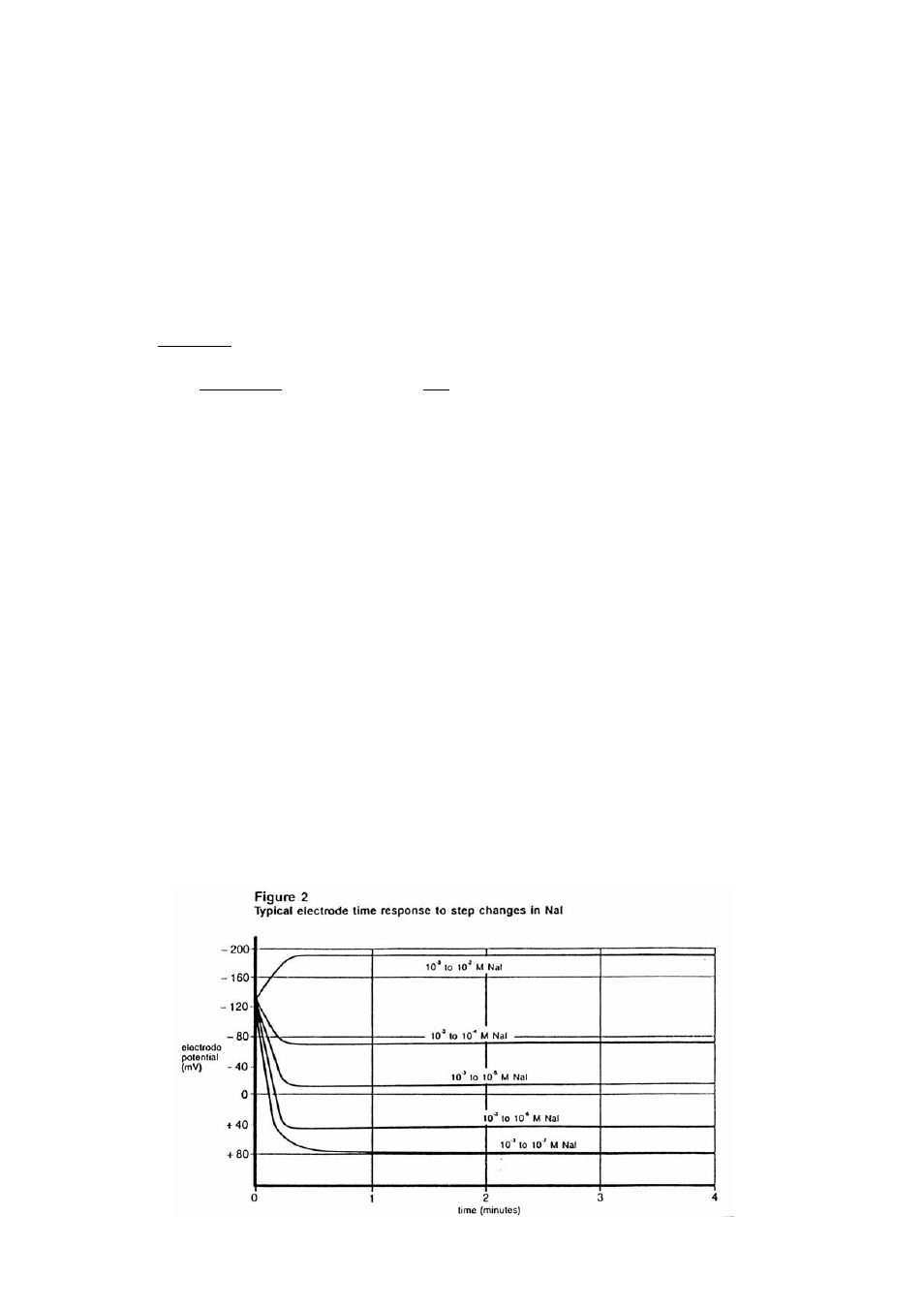
Electrode Response
Plotting the electrode mV potential against the iodide concentration on semi-logarithmic paper
results in a straight line with a slope of about 57 mV per decade. (Refer to Figure 1.)
The time needed to reach 99% of the stable electrode potential reading, the electrode response time,
varies from several seconds in highly concentrated solutions to several minutes near the detection
limit. (Refer to Figure 2.)
