Renata SA Chemistry and Construction User Manual
Chemistry and construction
38
www.renata.com
Chemistry and Construction
Chemistry of RENATA Li/MnO
2
cells
Renata CR lithium coin cells use a non-aqueous,
aprotic organic electrolyte containing lithium per-
chlorate in a mixture of organic solvents. The pro-
prietary formulation of the active cathode material
consists of a heat-treated mixture of electrolytic
MnO
2
and other specific components, yielding an
outstanding volume/capacity ratio for this Li/MnO
2
system.
The cell reactions for this electrochemical system
are:
Anode: Li –> Li
+
+ e-
Cathode: Mn
IV
O
2
+ Li
+
+ e- –> Mn
III
O
2
(Li
+
)
Overall cell reaction: Li + Mn
IV
O
2
–> Mn
III
O
2
(Li
+
)
Manganese dioxide is reduced from the
tetravalent to the trivalent state by lithium.
The separator system in Renata coin cells is
especially designed to ensure the best
performance in terms of mechanical strength, ion
permeability over a wide temperature range
(-40 to +100°C) and a low self-discharge rate.
Additional care in cell design also minimizes
self-discharge rate.
The combination of these several features
provides the best performance for long life
applications (back-up etc.)
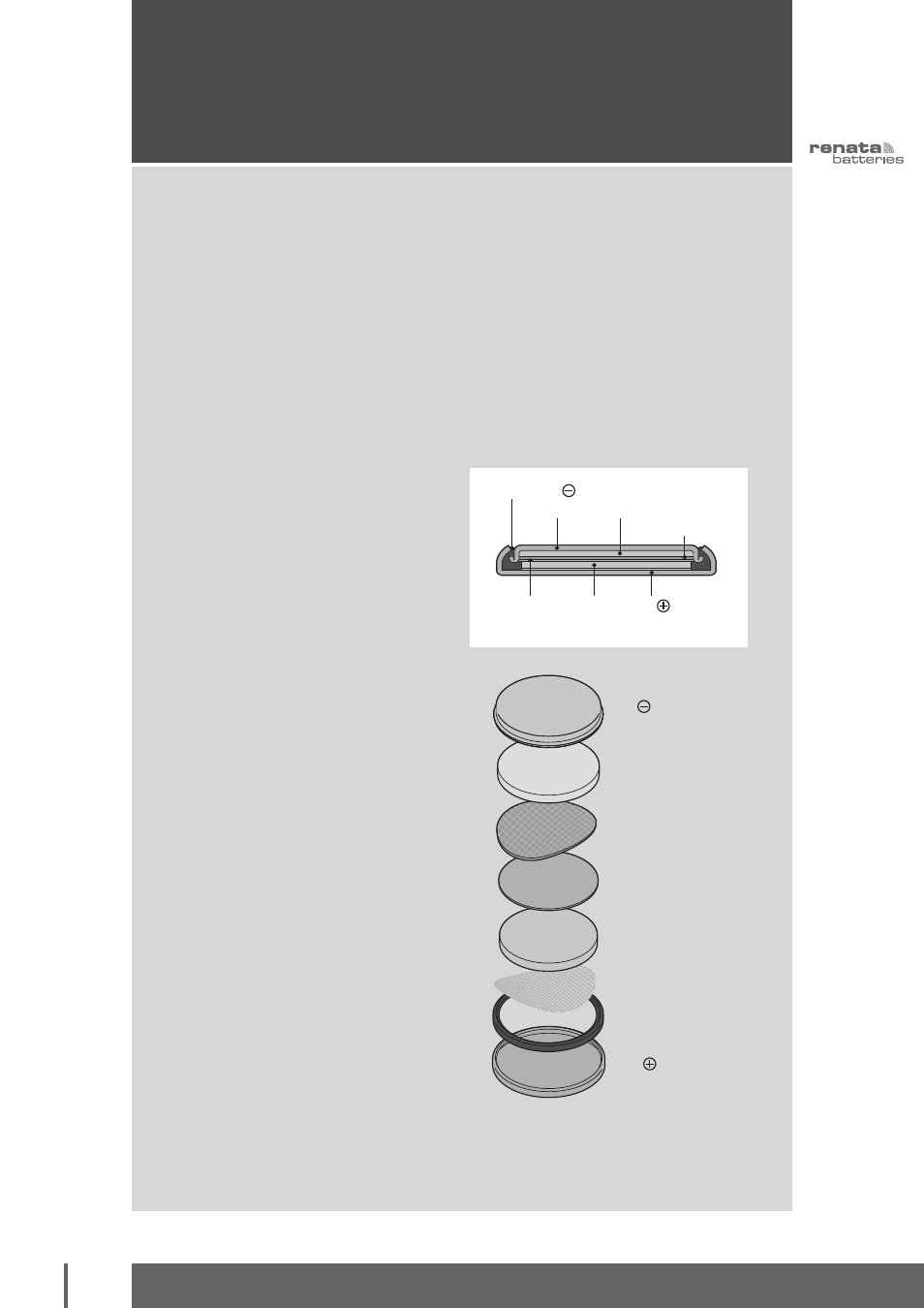
Separator
Gasket
Anode
(Lithium)
Absorbent
Layer and
Electrolyte
Cathode
(MnO
2
)
Cup
Contact
Lid
Contact
Separator
Gasket
Current Collector
Anode (Lithium)
Absorbent Layer and
Electrolyte
Cathode (MnO
2
)
Cup Contact
Lid Contact
Construction of RENATA Li/MnO
2
cells
