Ionode IJ-F Fluoride User Manual
Page 2
Application
The Ionode IJ-F Ion selective electrode responds reversibly
to Fluoride ions. The limit of detection is 0.02 ppm F-.
The response is theoretical down to approximately 0.2 ppm
Interfering Ions
The major interference is OH- . For best results, ensure
measurement is done in the pH range of 5 –7 pH (optimally
5.2-5.5pH). In common applications such as drinking water
analysis, this is easily achieved with the use of Tisab
(Total Ionic Strength Adjuster Buffer).
Cleaning
If the membrane is poisoned by interferences, the surface
may be carefully polishing with a fine polishing agent such
as 3
µm
diamond paste. Polishing the membrane gently with
fluoridated toothpaste can also restore sensitivity.
Organic contaminants can be removed with ethanol.
DO NOT use the electrode in chlorinated hydrocarbons.
Routinely remove the sleeve and replace the potassium
chloride electrolyte.
Electrolyte Replacement
The IJ-F has an inbuilt double junction Ag/AgCl with a
replaceable sleeve electrolyte. Saturated potassium chloride
is suitable for most applications.
Calibration Standards
Standard solutions of Fluoride Ion should preferably bracket
the expected measurement range. For example, to determine
Fluoride ions in drinking water (expected value around 1
ppm), it is normally sufficient to calibrate with 0.2 and 2.0
ppm Fluoride standards.
Calibration Procedure
Make up F- calibration standards in distilled water bracketing
the expected measurement range. Starting with the lowest
concentration first, measure out 20ml of standard and add
20ml Tisab. Insert the electrode after rinsing , stir, and note
the potential in mV. Perform this (carefully rinse between
measurements to eliminate carry over) on each of the standards.
At room temperature, a slope should be obtained of around
-55mV/decade. (from 0.2ppm upwards)
Sample Measurement
Follow the same basic procedures as calibration,
substituting the calibration standard for your sample.
It is important to use the same stirring conditions,
temperature, etc for best results. Use the same ratio
of sample/ISA as used in the calibration step.
Read off the concentration from the graph or directly
from Ion meters.
Visit ionode.com for more details.
Introduction
This guide contains the basic information for proper use
of your new Fluoride Ion-selective electrode.
Preparation
IJ series electrodes are shipped without sleeve electrolyte,
and must be filled prior to use. To fill, hold the electrode
by the sleeve and gently ease off the rubber wetting cap.
Prepare as follows:
2.
Fill the annular space with gel
or electrolyte to approximately
half to three quarter full.
1.
2.
1.
Invert the electrode. Hold the
electrode just below the sleeve
and with careful rotation and
pulling along the axis of the
electrode, remove the sleeve.
DO NOT BEND.
3.
Slide the sleeve back onto the electrode ensuring the black
O-ring is well seated within the electrode body. Do not exert
sideways force. Any excess electrolyte will be expelled from the
end of the electrode through the ground junction. Ensure there
are no air bubbles in the sleeve.Wash off any excess electrolyte
before use.
3.
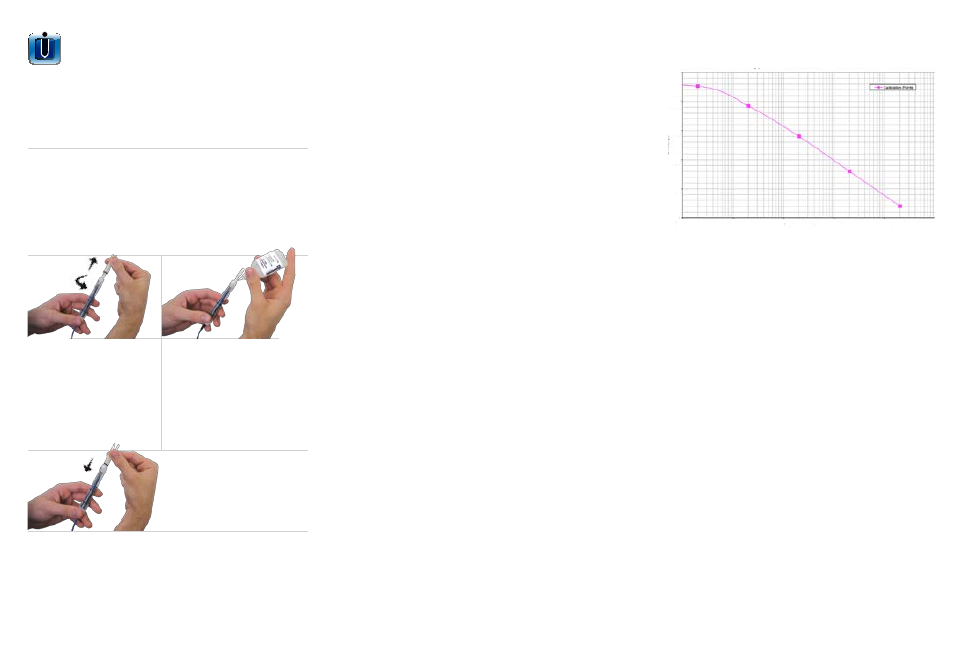
Typical Calibration Curve
Concentration PPM
250
200
150
100
50
0
0.01
0.1
1
10
100
P
ot
ential mV
GOOD CHEMISTRY
GOOD CHEMISTRY
