Elenco Solar Energy User Manual
Page 8
7
Experiment no. 9 The solar pond
At the southern tip of Israel, near the town of Eilat, is a small lake. At first glance
nothing seems remarkable about it. The water is warm and salty and not particularly
clean. However, this lake attracts scientists from all over the world because it is a
“solar pond.” If current research proves successful, this small lake may make a major
contribution towards solving the world’s energy shortage.
If the sun shines on a pool of water, the top layer becomes slightly hotter since hot
water usually rises to the top. But hot water evaporates faster than cold, and when
water evaporates, it takes heat energy with it.
You can easily test this by wetting your forearm and blowing on it. When you blow on
your wet arm, you speed the process of evaporation. You arm feels cold during this
process because heat is lost from your arm while the water evaporates. The water has
used the heat from your arm as a part of the evaporation process.
In an ordinary pool the sun shines on the water, adding heat. Then the water
evaporates, losing heat. Eventually, a balance is reached where any extra heat causes
more evaporation and the pool reaches a steady temperature.
A solar pool is different. Underground there is a salt-water well so the water at the
bottom is much more salty than the layer at the top. The top layer acts as a magnifying
glass, focusing the sun’s heat onto the lower layer. The salty water is heavier than
ordinary water, even when hot. Therefore, unlike ordinary water, the heated salty
water sinks, instead of rising. It is now strictly prohibited to bathe in this pool. Before
the scientific facts relating to this solar pond were known, swimmers who dived into the
bottom were badly scalded. Today, scientists are investigating the possibility of building
artificial solar pools in order to exploit this solar energy trapped in the salt-water layer.
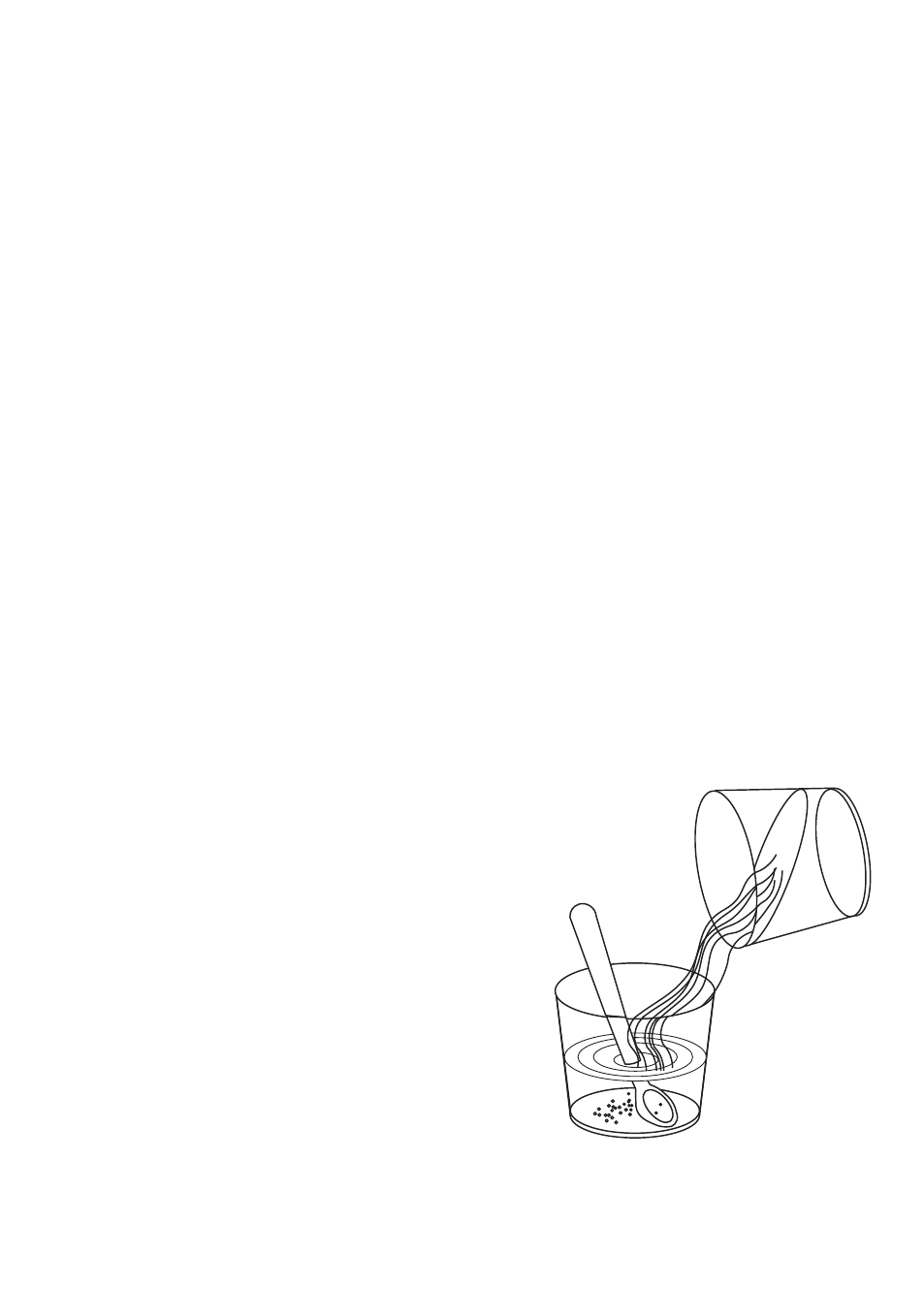
You too can try out this idea. Take a coloured plastic
bowl or glass (not white.) Fill it to one-third of its
depth with water and put in as much salt as will
dissolve. If you have some ink or dark food dye,
colour this solution.
Now, carefully and slowly pour into the bowl or glass
some fresh water. Pour it slowly down one side,
using a spoon to direct the flow. It is important to
prevent the two liquids from mixing. The ink in the
salt water will tell you if you have been successful.
Let it stand in the sun for some time and then measure
the temperature at the top, the middle and the bottom.
concentrated
salt water
and ink
fresh water
