C.B.S. Scientific ECU-040 User Manual
Page 8
C.B.S. Scientific
Electro-Eluter Concentrator
8
SECTION 3
Instructions for Use
3.1 Unit Preparation
Place the Electro-Eluter Concentrator and the Elution blocks on a level work surface in an
authorized work area.
3.2
Elution Block Set-Up
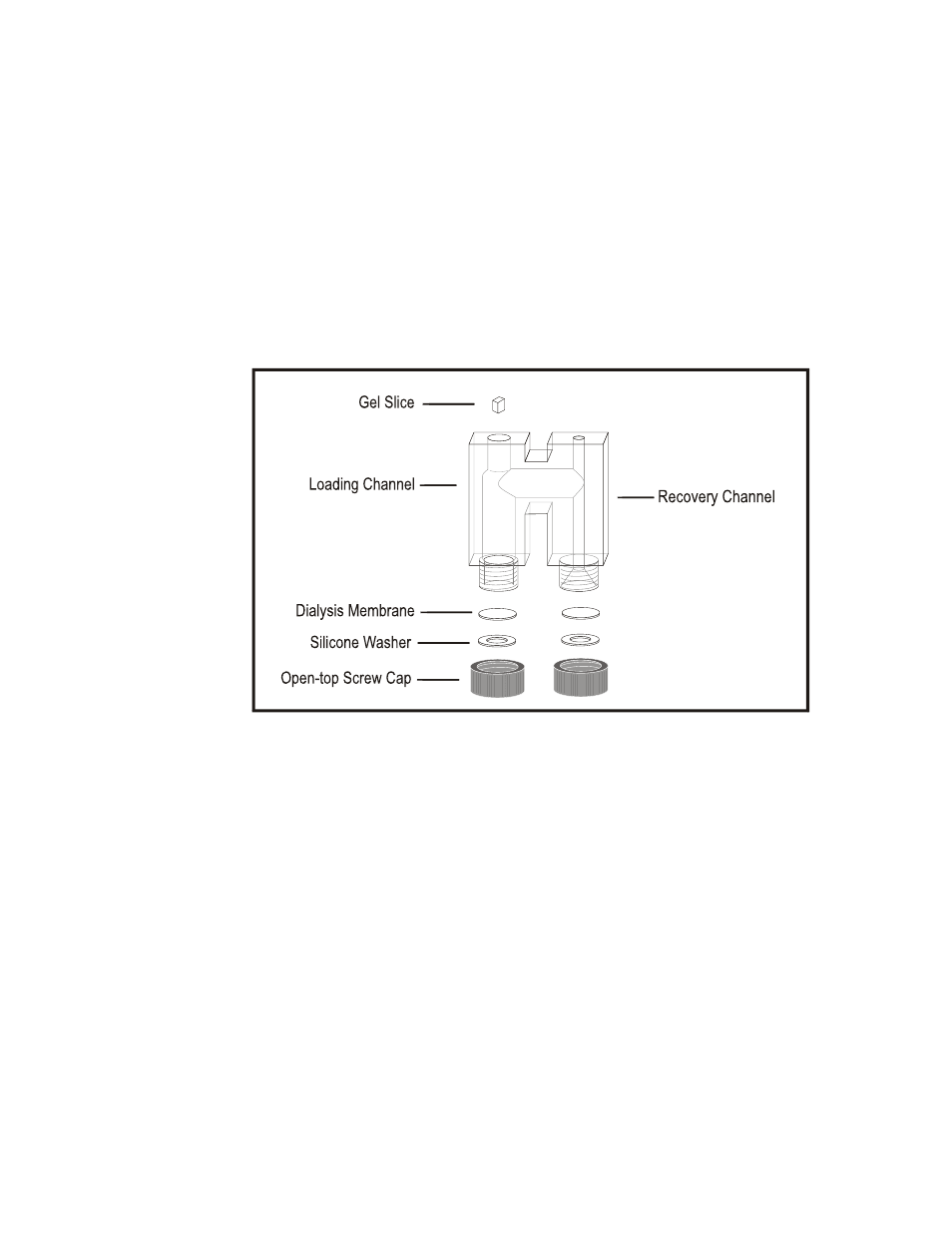
To assemble Block for electroelution, unscrew black caps and remove washers. Cut dialysis
membrane into circles the same diameter as the Teflon/Silicone Washers. Place the membrane
onto the slightly tan side of the washer inside the black cap (the Teflon will allow the membrane to
slide instead of binding on the washer). Screw the black cap with the washer and membrane into
place as shown in figure 1.
3.3
Procedure for Protein Elution
1.
For electroelution of protein samples prior to sequencing, read carefully the Hunkapillar et. al.
paper in Methods in Enzymology, Vol. 91, 1983, pgs. 227-236.
2.
Equilibrate each block with 1% Ethanolamine to take away most of the non-specific binding
sites for protein attachment. The Plexiglas walls of the blocks, if not treated, may decrease
the yield from electroelution by non-specific absorption. Dilute the Ethanolamine in running
buffer.
3.
The gel slice should be equilibrated in running buffer, as per the Hunkapillar paper. On the
large volume side of the Block, top load the gel slice onto the membrane. Fill the entire
chamber with running buffer ¾ of the way up to the cross channel. Tap blocks with finger so
that ALL THE AIR BUBBLES TRAPPED INSIDE ARE DISLODGED.
4.
Insert loaded blocks into unit. Flush air trapped beneath each black cap with syringe and
bent needle. Be careful not to puncture membrane.
