Eppendorf Microinjection of cDNA into Drosophila embryos User Manual
Page 3
Freshly hatched y1w1118 flies with an integrase target site
(attP) and a phi-integrase source on the 4
th
chromosomes
were transferred to an egg laying cage 2-3 days prior to
injection in order to acclimatize them to the condition.
The microinjection preparation has the following scheme:
1. Let female lay for 30-40 min on freshly yeasted grape
juice agar plates in the dark at RT.
2. Change the plates and treat the laid embryos with so-
dium hypochlorite diluted in water in a 1:4 ratio for 2 min to
remove the chorion.
3. Collect the dechorionated embryos with a mesh basket
and wash thoroughly with water to remove traces of bleach.
4. Line up approximately 100 embryos at the edge of an
agar block with their anterior poles facing outwards.
5. Press carefully a microscope slide with a stripe of
double-sticky tape on the lined-up embryos and dry them
on the microscope slide with a hair-dryer with cool-shot
function.
Note: Adjust conditions (length of drying and distance to
the air stream) carefully in advance depending on the room
temperature and humidity.
6. Cover embryos with Voltalef 10S oil and inject them with
the DNA mixture at the posterior poles.
DNA used for injection is prepared with Qiagen Midiprep
kit, resuspended in injection buffer (5 mM KCI, 0.1 mM
NaH
2
PO
4
, pH 6.8).
The DNA to be injected is quantified and diluted at a
concentration of 0.3 µg/µL in a final volume of 20 µL. The
mixture is centrifuged at 20,000 x g for 15 min at RT (room
temperature) followed by additional 15 min at 4 °C to re-
move particles that may block the microinjection needle.
Userguide No 041 | Page 3
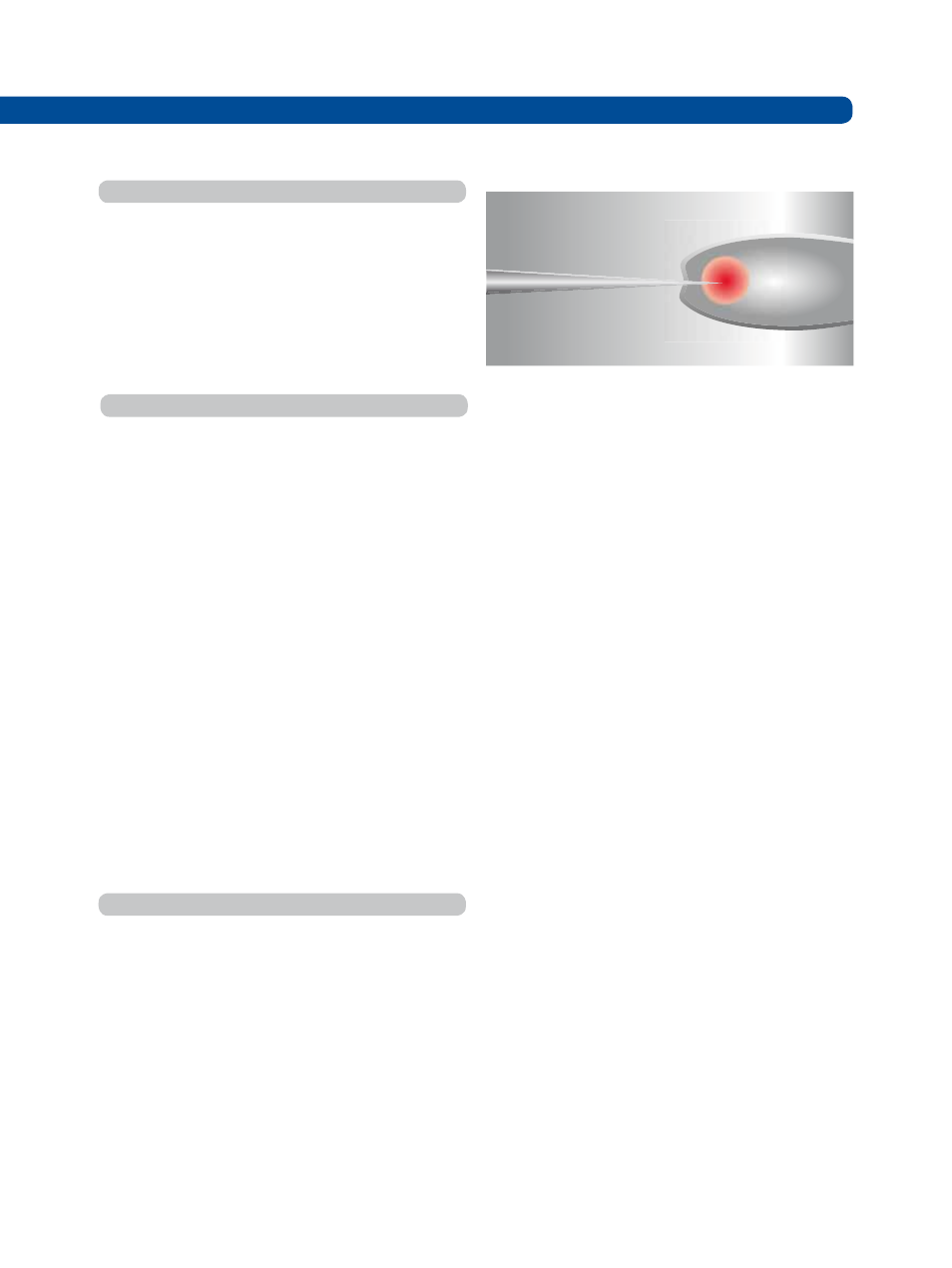
Fig. 2: Injection into the posterior of an embryo, injection is high-
lighted with red droplet
by hand control (see figure 1), left mouse click induces injec-
tion pressure, right mouse click triggers the Clean function.
Another option is to trigger the injection by using a foot con-
trol. If the sample preparation was performed as described
above, the capillary is not blocked by particles and the
injection can be performed also with the continuous flow of
a high adjusted compensation pressure (Pc) of the FemtoJet
microinjector and constant moving the embryo against the
needle.
Note: Injection parameters need to be adjusted prior each
experiment, but we suggest as starting and optimal parame-
ters an injection pressure (Pi) around 170 hPa and a compen-
sation pressure around 200 hPa. The latter can be increased
up to 300 hPa if necessary.
3. The embryo is successfully injected when a droplet of
DNA is seen to diffuse in the embryo.
Trouble shooting:
In case the injection of some embryos is not successful
with the constant flow, use the injection button or hand con-
trol to trigger injection.
In case the needle gets clocked or stuck press the button
Clean. With the Clean function button or the right mouse-
click of the hand control, a pressure of 6000 hPa is applied
as long as the button is pressed.
After an injection session is finished carefully detach the
tape stripe with embryos and transfer it onto agar plates
with baker’s yeast in a humidified chamber.
After 2 days at 18 °C transfer the plates to a 25 °C incubator
until pupation occurred. Pupae were then carefully trans-
ferred into fresh fly tubes.
DNA preparation
Preparation of the Drosophila embryos
Injection procedure
1. Focus on the embryo on the microscope slide at one end
of the line.
2. Fill the capillary bubble-free with the aid of Microloaders,
mount it in the holder and bring the tip of the needle as close
as possible to the first embryo in order to inject the embryo
(according to figure 2). In our laboratory, the injection ist trig-
gered by using the respective buttons on the device or
