Myron L 3P User Manual
Page 12
the lifetime of a pH junction from depletion of solution inside the
reference junction or from contamination. The junction is damaged by
drying out because insoluble crystals may form in a layer, obstructing
contact with test solutions. See Cleaning Sensors, pg. 16.
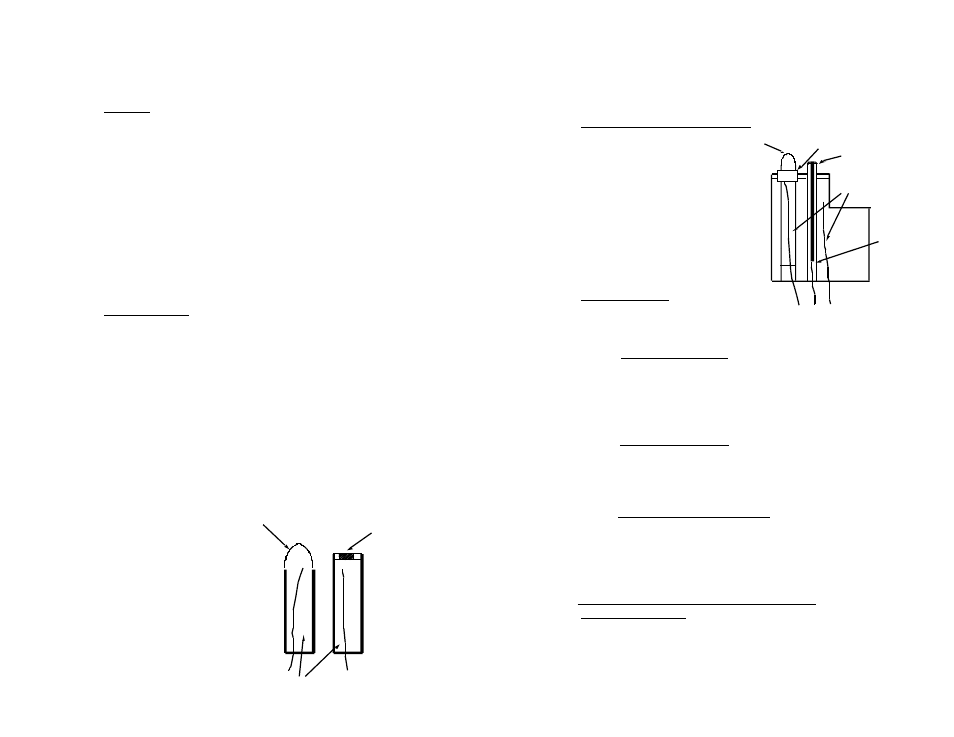
4. The Myron L Integral pH Sensor
The sensor in the Ultrameter (Figure 10)
is a single construction in an easily
replaceable package. The sensor body
holds an oversize solution supply for long
life. The reference junction “wick” is
porous teflon to provide a very stable,
low permeability interface. It is formed
in a ring around the pH sensing electrode.
The construction combines all the best
features of any pH sensor known.
5. Sources of Error
The basics are presented in Cleaning
Sensors, pg. 16.
a. Reference Junction
The most common sensor problem will be a clogged junction because a
cell was allowed to dry out. The symptom is a drift in the “zero” setting at 7
pH. This is why the Ultrameter does not allow more than 1 pH unit of offset
during calibration. At that point the junction is unreliable.
b. Sensitivity Problems
Sensitivity is the receptiveness of the glass surface, which can be
diminished by a film on the surface, or a crack in the glass. These
problems also cause long response time.
c. Temperature Compensation
pH sensor glass changes its sensitivity slightly with temperature, so the
further from pH 7 one is, the more effect will be seen. A pH of 11 at 40°C
would be off by 0.2 units. The Ultrameter senses the cell temperature and
compensates the reading.
B. ORP/Oxidation-Reduction Potential/REDOX
1. ORP as an Indicator
ORP is the measurement of the ratio of oxidizing activity to reducing
activity in a solution. It is the potential of a solution to give up electrons
(oxidize other things) or gain electrons (reduce).
21
Junction plug
Platinum button
Figure 10
H+ ions
Electrode wires
KCl solution
Glass
Sleeve
Glass
Surface
In a solution of one known component, pH will indicate concentration
indirectly. However, very dilute solutions may be very slow reading, just
because the very few ions take time to accumulate.
2. pH Units
The acidity or alkalinity of a solution is a measurement of the relative
availabilities of hydrogen (H ) and hydroxide (OH ) ions. An increase in
(H ) ions will increase acidity, while an increase in (OH ) ions will increase
alkalinity. The total concentration of ions is fixed as a characteristic of
water, and balance would be 10 mol/liter (H ) and (OH ) ions in a
neutral solution (where pH sensors give 0 voltage).
pH is defined as the negative logarithm of hydrogen ion concentration.
Where (H ) concentration falls below 10 , solutions are less acidic than
neutral, and therefore are alkaline. A concentration of 10 mol/liter of (H )
would have 100 times less (H ) ions than (OH ) ions and be called an
alkaline solution of pH 9.
3. The pH Sensor
The active part of the pH sensor is a thin glass surface which is selectively
receptive to hydrogen ions. Available hydrogen ions in a solution will
accumulate on this surface and a charge will build up across the glass
interface. The voltage can be measured with a very high impedance
voltmeter circuit; the trick is to connect the voltmeter to solution on each
side.
The glass surface encloses a captured solution of potassium chloride
holding an electrode of silver coated with silver chloride. This is as inert a
connection as can be made from metal to an electrolyte. It still can
produce an offset voltage, but using the same materials to connect to the
solution on the other side of the membrane allows the 2 equal offsets to
cancel.
The problem is...the other side of the
membrane is some test solution,
not potassium chloride. The outside
electrode, also called the Reference
Junction, is of the same construction
with a porous plug in place of a glass
barrier to allow the junction fluid to
contact the test solution without
significant migration of liquids
through the plug material. Figure
9 shows a typical 2 component pair.
Migration does occur, and this limits
20
-
+
-
+
-
7
+
-
Figure 9
KCl solution
Electrode wire
Glass surface
Junction plug
Electrode wire
H+ ions
-
7
-
9
+
+
+
-
