Introduction, Conductivity and ph: how they can help you – Myron L Litho-Kit User Manual
Page 3
INTRODUCTION
Your Litho-Kit™ is a quality control "tool" to help you
print better. It and this Guide will improve your printing
through better control of fountain (dampening) solutions.
The rising popularity of alcohol-free solutions has
increased the need for very careful monitoring of their
conductivity, pH and temperature.
While this Guide offers information we hope will be very
useful, it makes no specific recommendations regarding
fountain solution temperature, concentration, pH or
conductivity values. A good source for such information
is your solution supplier, who is most familiar with
your local conditions. Another source is the Graphic
Arts Technical Foundation, a non-profit research and
educational organization which provided much of the
information in this Guide.
CONDUCTIVITY AND pH: HOW THEY CAN
HELP YOU
The Myron L instrument which is the "heart" of your kit
is either a conductivity instrument or a conductivity/pH
instrument. Both are industrial-quality instruments for
professionals. Reliable even in demanding conditions, they
feature electrodes mounted inside a cell cup for maximum
protection. Details of specifications and operation can be
found in the instruction manual in each kit.
Conductivity is the ability of a solution to pass an
electrical current. The amount of current passed
depends on the concentration of ions, or electrically
charged particles in the solution. The higher the
concentration of ions, the higher the degree of
conductivity. The unit of conductivity measurement is the
microsiemen (also called the micromho).
Traditionally, pH, a measure of the degree of acidity
or alkalinity, was used to check fountain solution
concentration. Today, however, conductivity testing
is recognized as a much more accurate method.
Many modern dampening solutions are pH stabilized
(or buffered), so only small changes in pH are seen,
even when solution strength is dramatically changed.
The conductivity, however, increases as solution
concentration rises.
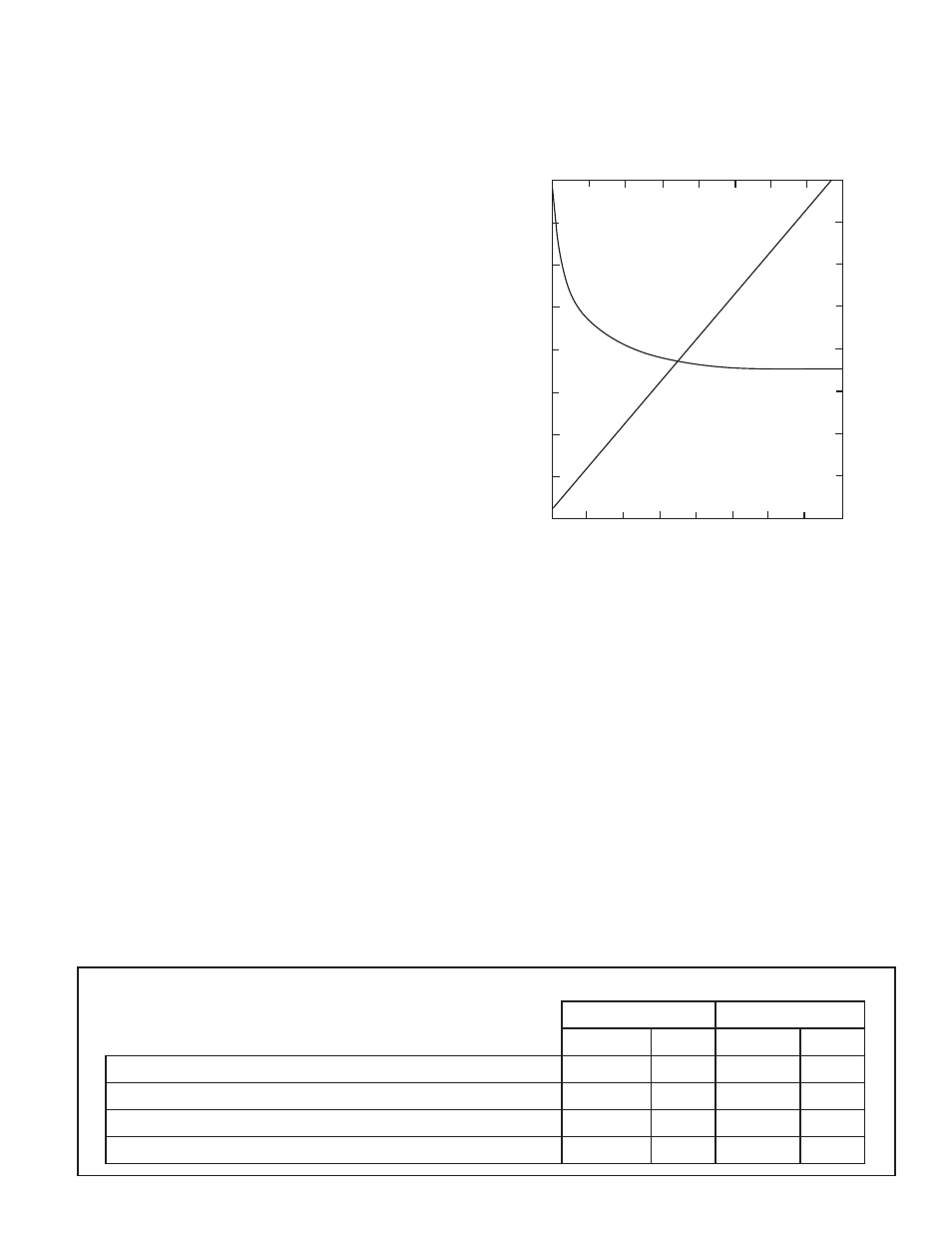
The advantage of checking fountain solution
concentration with conductivity, rather than pH, can be
seen in the following graph.
Concentration vs. pH and Conductivity for a hypothetical
combination of fountain solution concentrate and water
Notice how the pH levels off, but conductivity values rise
on a straight-line basis as the concentration increases.
This "linear" relationship allows you to easily match the
conductivity value to a specific concentration of your
own solution.
Even though pH usually is not the best method to check
the concentration of fountain solution, it is still very
important and must be checked regularly. The pH of acid
dampening solution affects sensitivity, plate-life, ink-
drying, etc. Also, pH can change during a run if the paper
has a high acid or alkaline content. Conclusion: pH must
be maintained at the proper level for good printing.
The table below lists recommendations for checking
fountain solution conductivity and pH.
C
onduc
tivit
y (micr
omhos/cm)
pH
pH
Conduc
tivit
y
Concentration (oz./gal.)
3500
3000
2500
2000
1500
1000
500
0
1
2
3
4
5
6
7
8
7
6
5
4
3
2
1
RECOMMENDED TESTING METHOD
MIXING
ON PRESS
TYPE OF FOUNTAIN SOLUTION
COND.
pH
COND.
pH
ACID
X
X
X
X
BUFFERED ACID
X
X
X
NEUTRAL
X
X
X
ALKALINE
X
X
X
