Thermo Fisher Scientific Ion Selective Electrodes Fluoride User Manual
Page 13
Instruction Manual
Fluoride Electrode
- 10 -
where:
x
Cs = concentration of sample
o
Cs = fluoride standard concentration (0.1M)
x
Vt = volume of titrant added to achieve the endpoint of unknown sample
Vt = volume of titrant added to achieve the endpoint in standardization
x
Vf = volume of sample used in sample titration
Vf = volume of standard used in standardization titration.
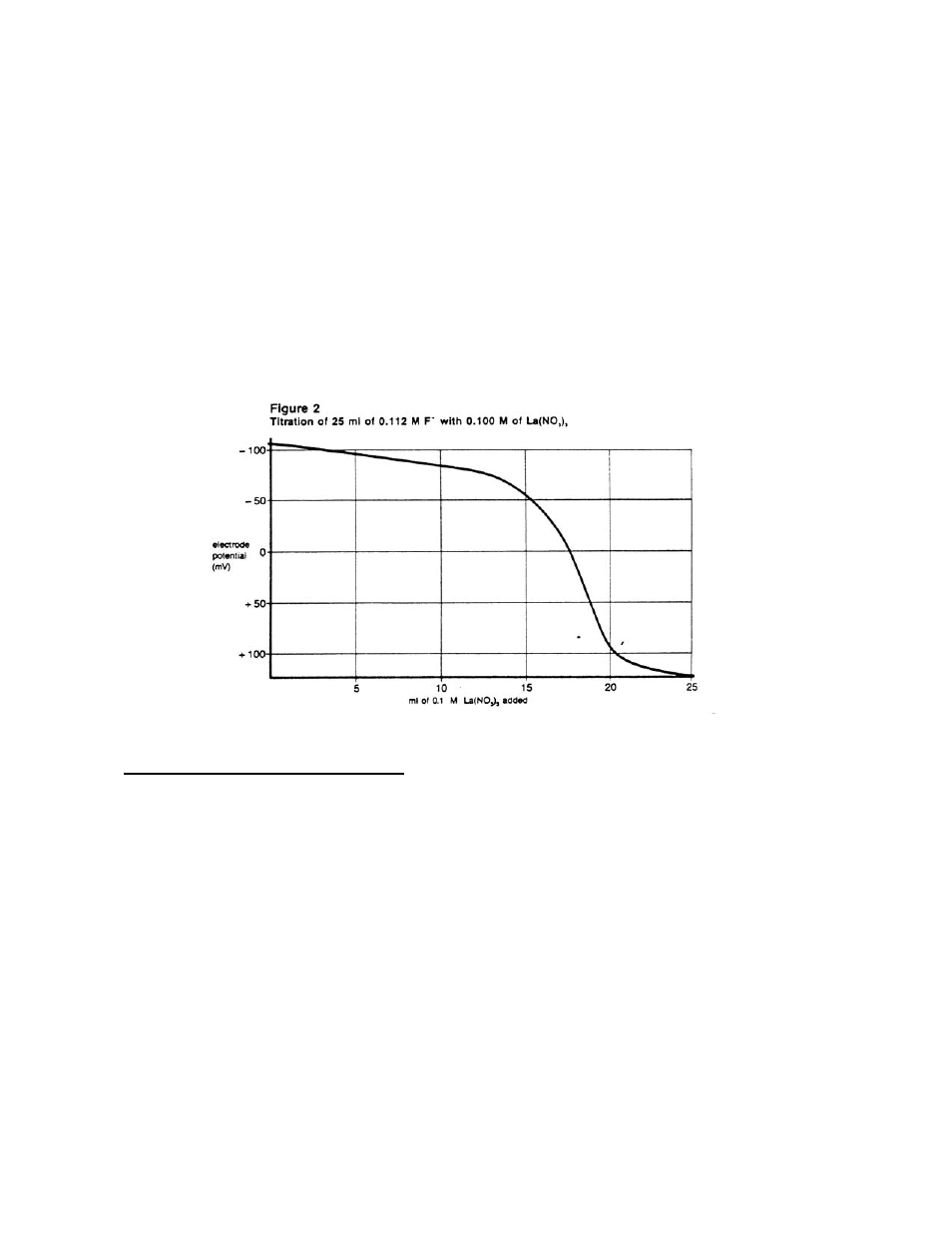
A typical titration curve is shown in Figure 2.
ELECTRODE CHARACTERISTICS
Reproducibility
Electrode measurements reproducible to
±2% can be obtained if the electrode is calibrated every
hour. Factors such as temperature fluctuations, drift, noise, and variations in illumination limit
reproducibility. Reproducibility is independent of concentration within the electrode's operating
range.
Interference
The hydroxide ion, OH
-1
, is an electrode interference. Anions which make the sample more basic,
such as CO
3
-2
or PO
4
-3
would increase the OH
-1
interference, but do not interfere with direct
electrode operation. Other anions commonly associated with fluoride, such as Cl
-1
, Br
-1
, I
-1
, SO
4
-2
,
HCO
3
-1
, NO
3
-1
, and acetate, do not interfere with correct electrode operation. Most cations do not
interfere with the response of the fluoride electrode to fluoride ion.
